
Food Research International 149 (2021) 110655
Available online 20 August 2021
0963-9969/© 2021 The Author(s). Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Review
Design of polyphenol-rich diets in clinical trials: A systematic review
Luis Condezo-Hoyos
a
,
b
, Christina Gazi
a
, Jara P
´
erez-Jim
´
enez
a
,
*
a
Department of Metabolism and Nutrition, Institute of Food Science, Technology and Nutrition (ICTAN-CSIC), Madrid, Spain
b
Universidad Nacional Agraria la Molina, Facultad de Industrias Alimentarias, Innovative Technology, Food and Health Research Group, La Molina, Lima, Perú
ARTICLE INFO
Keywords:
Polyphenols
Clinical trial
Systematic review
Healthy diet
ABSTRACT
Most randomized clinical trials of polyphenols focus on individual foods. Nevertheless, due to their presence in
many foods and in order to reect a real situation, clinical trials based on polyphenol-rich diets are particularly
important. This systematic review explores the characteristics of the polyphenol-rich diets used in intervention
studies. The bibliography search for English-language scientic papers was performed in the Elsevier Scopus
Database and PUBMED in March 2020, and focused on intervention studies with whole polyphenol-rich diets,
establishing several exclusion criteria. In studies fullling the requirements, information on the design of the
polyphenol-rich diet and associated polyphenol intake was extracted and compared. A total of 5 studies were
selected. Among them, substantial differences were found in the design of the polyphenol-rich diets, regarding
specic instructions and concerning the foods provided. Similarly, although a median daily polyphenol intake of
2,564 mg/day (17,945 mg/week) was obtained from the studies, which corresponds to a nutritional dose, intake
values varied widely both for total polyphenols (the difference between studies reached threefold), and for in-
dividual polyphenol intake (for hydroxycinnamic acids, a tenfold difference was found between percentile 25
and percentile 75 values). These differences made the comparison of results difcult and may affected the
observed health effects. Thus, despite the relevance of studying polyphenol-rich diets as a whole, this systematic
review found substantial differences between the studies performed, making direct comparisons difcult.
1. Introduction
Polyphenols constitute a large group of plant secondary metabolites
widely distributed throughout the plant kingdom (Rodriguez-Mateos
et al., 2014). They exhibit several biological activities, for instance
functioning as antioxidants (Morvaridzadeh et al., 2020a) and anti-
inammatory compounds (Morvaridzadeh et al., 2020b). Cumulative
scientic evidence, including the results of mechanistic experiments,
assessments of metabolic fate, clinical trials and observational studies,
has shown that these compounds play a promising role in the modula-
tion of several non-communicable chronic diseases, particularly car-
diometabolic diseases (Serino & Salazar, 2018; Martini et al., 2019), as
well as certain kinds of cancer (Bondonno et al., 2019), neurodegener-
ative processes (Squillaro et al., 2018) and specic diseases such as
polycystic ovarian syndrome (Heshmati et al., 2020).
However, research on polyphenols has sometimes apparently been
contradictory as demonstrated, for instance, in two systematic reviews
of the role of grapes (one of the fruits with the highest polyphenol
content) in the regulation of metabolic syndrome (Akaberi & Hossein-
zadeh, 2016; Woerdeman et al., 2017). It has been suggested that con-
sistency in several aspects when designing and performing nutritional
clinical trials would eliminate some of these discrepancies; for instance,
clearly dening clinical endpoints when translating preclinical studies
on polyphenols into clinical trials (Nú
˜
nez-S
´
anchez et al., 2015). In the
same way, some studies have suggested certain criteria for reporting
results of inter-individual variability in the response to bioactive dietary
compounds, a highly relevant aspect in the eld of polyphenols (Nikolic
et al., 2019), as well as for study design and analytical determinations in
studies on the biological effects of bioactive compounds, including
polyphenols, on gene expression (Pokimica & García-Conesa, 2018).
Also, the importance of systematically measuring microbiota variations
in clinical trials with polyphenols has been addressed (Marino et al.,
2020). Overall, the increasing interest in recommendations on how
polyphenol studies should be conducted or reported shows the relevance
of study design and how it is an aspect that deserves specic attention.
Another relevant aspect is that many clinical trials with polyphenols
* Corresponding author at: Department of Metabolism and Nutrition, Institute of Food Science, Technology and Nutrition (ICTAN-CSIC), Jos
´
e Antonio Novais 10,
28040 Madrid, Spain.
E-mail address: [email protected] (J. P
´
erez-Jim
´
enez).
Contents lists available at ScienceDirect
Food Research International
journal homepage: www.elsevier.com/locate/foodres
https://doi.org/10.1016/j.foodres.2021.110655
Received 17 March 2021; Received in revised form 27 July 2021; Accepted 17 August 2021
Food Research International 149 (2021) 110655
2
have evaluated the effect of supplementation with an individual food
(Akaberi & Hosseinzadeh, 2016; Gianfredi et al., 2018; Woerdeman
et al., 2017). Although such studies are absolutely needed for the
identication of responses to individual foods, at the same time the ef-
fect of the whole diet may not be disregarded. Indeed, it has been
pointed out that the combination of polyphenols from different foods is
precisely what can give rise to biological activities (de Pascual-Teresa &
Clifford, 2017). And a study (Molinar-Toribio et al., 2018) where rats
consuming either a control diet of a high-fat high-sucrose diet were
supplemented with grape seed extract showed that the generation of
microbial-derived metabolites was decreased in the high-fat high-su-
crose group, probably due to a shift in microbial communities. This
might show modied biological activities of a particular polyphenol
supplementation in the context of an unhealthy dietary pattern. For
these reasons, some researchers have chosen to evaluate the effect of a
polyphenol-rich diet (Bozzetto et al., 2015; Noad et al., 2016); they have
shown benecial effects in subjects at high cardiometabolic risk, which
has increased the existing evidence on the biological relevance of
polyphenols. Nevertheless, the issue of heterogeneity in the design also
arises here, for example: What may be considered a polyphenol-rich
diet? Which individual foods are included? In what proportions? How
detailed must the instructions provided to the volunteers be? All these
aspects are relevant when it comes to comparing results, as well as in
order to establish public health recommendations; but to date, they have
not been explored in detail.
For these reasons, we consider it is pertinent to perform a systematic
review of nutritional clinical trials based on polyphenol-rich diets. The
aim of this systematic review is not to address a specic clinical ques-
tion, since the selected studies may have dealt with different physio-
logical situations and focused on varied primary outcomes, but to focus
on the concept of a polyphenol-rich diet itself and how it has been
dened in previous studies (foods, serving sizes and associated poly-
phenol intake), in order to identify the main shared characteristics and
divergences between them and, ultimately, to suggest criteria that will
allow consistency in this kind of study. Therefore, the question we
address here is: “How are polyphenol-rich diets designed in nutrition
clinical trials?” We adopted an established PICOS approach, as dened
below. Quantitative data on polyphenol content in the designed diets
were integrated from the different studies and an overall analysis was
carried out; nevertheless, since these data did not correspond to modi-
cations in a clinical outcome, this not a meta-analysis.
2. Methods
This systematic review was conducted according to PRIMSA guide-
lines; although, as it did not deal with a specic clinical question, some
aspects could not be adopted. The complete PRISMA checklist is pro-
vided as Supplementary Table 1.
2.1. Search strategy and study selection
The bibliographic databases Elsevier Scopus Database and PUBMED
were systematically searched for relevant papers until March 2, 2020.
No other sources were included.
The following PICOS parameters were applied: Participants, adults;
Intervention, treatment with polyphenol-rich diet; Comparison, inter-
vention treatment with low-polyphenol diet; Outcome, since the focus of
the systematic review was the design of the polyphenol-rich diet, no
clinical outcome was predened; Study design, only intervention studies
were selected.
The rst step of the systematic search was performed with the
following keyword combinations “polyphenol*” AND “diet” AND
(“trial” OR “intervention”) AND “human” and the papers returned were
exported to Endnote software (Clarivate Analytics, PA, USA) with
duplicated papers being eliminated. Once in Endnote, the titles of papers
were screened for two inclusion criteria: I) trial OR intervention AND
diet; and II) poly* OR av* OR phe*. After this, the abstracts were
analyzed based on the following inclusion criteria: 1) an original study;
2) conducted in humans; 3) an intervention study, including either
randomized controlled trials (2 arms with and without intake of a
polyphenol-rich diet) or before-and-after studies (one arm following a
polyphenol-rich diet for a certain period); 4) evaluating the effect of a
polyphenol-rich diet; 5) published in English; and 6) data presented in a
usable format. Conversely, the following exclusion criteria for this step
were dened: 1) reviews; 2) preclinical studies; 3) observational studies;
4) studies based on supplementation with a single food or additive; 5)
languages other than English; 6) absence of extractable data (see below).
2.2. Quality assessment
The JADAD score evaluation system was applied for assessment of
the methodological quality of the 5 clinical trials selected (Reis et al.,
2019). The studies were scored according to key methodological fea-
tures: randomization (R), sequence of randomization (SR), blinding (B),
method of blinding (MB), withdrawals/dropout (WD), inappropriate
randomization (IR) and inappropriate blinding (IB) (Bisol et al., 2020).
The studies with a JADAD score ≥ 3 were considered to have high-
quality methodology (Bisol et al., 2020).
2.3. Data collection
Data were extracted from the clinical trial studies selected with a
JADAD score ≥ 3; although characteristics of the study design, such as
randomization, would not affect the results of the present systematic
review, focused on diet design, at the same time it was considered that
the higher the quality of the overall study design was, the higher the
probability for the existence of a detailed and justied polyphenol-rich
diet was. The selected studies included: bibliographic data, study char-
acteristics (aim, design, and location), participants (age, sex, and char-
acteristics), and a description of the dietary intervention. Since this last
aspect is the main point of interest of this systematic review, the
following information was collected, when it was available: 1) specic
intervention concept (polyphenol-rich diet, avonoid-rich diet and
others); 2) food groups included or excluded in the diet; 3) individual
foods included or excluded in the diet; 4) instructions about number and
size of servings per day or week; 5) degree of detail in the instructions
(specic meals to be consumed, and general lists of recommended
foods); 6) expected polyphenol intake of the subjects and degree of
detail (by class or individual compounds); 7) actual polyphenol intake of
the subjects and degree of detail (by class or individual compounds); 8)
source of polyphenol content information (in-house data or public da-
tabases); and 9) follow-up strategy for assessing dietary adherence.
2.4. Data processing
The information provided on polyphenol intake in the selected
studies was quite varied: in some cases, no information on polyphenol
intake was provided (Malaveille et al., 2004), while other studies
specied the value of total polyphenol intake (Della Peppa et al., 2020)
or avonoid content in the recommended foods (Chong et al., 2013).
Due to this diversity, the only way to process all the studies together and
obtain data on individual and total polyphenol intake, in order to
address the question we have proposed for this systematic review, was to
calculate the polyphenol intake for all the studies based on the infor-
mation they provided on the study design. To do this, we used the
validated Phenol-Explorer (Neveu et al., 2010) and USDA (Bhagwat &
Haytowitz, 2007) databases, which provide composition data for thou-
sands of individual foods from the literature, and we took into account
the amount of individual foods consumed weekly. When the polyphenol
content of individual foods was not available in these databases, specic
research papers were used to obtain the missing information, as was the
case of rocket (Santos et al., 2014), blueberry jam (
´
Scibisz & Mitek,
L. Condezo-Hoyos et al.
Food Research International 149 (2021) 110655
3
2007), cherry tomatoes (Je
˙
z et al., 2018) and watercress (Giallourou
et al., 2016).
In the case of the study by Chong et al., (Chong et al., 2013), since the
subjects received an additional polyphenol dose on top of their basal
intake, which was not indicated in the study, this was taken from the
quintile with the lowest polyphenol intake (since these subjects were
characterized by a low fruit and vegetable intake) determined in a
similar cohort. Furthermore, since the subjects were instructed to
consume a certain amount of fruit and vegetables distributed in several
items, but without further instructions, it was assumed that all the food
items were consumed in the same proportion. Finally, in that same study
the weight (g) was considered equal to volume (mL) for liquid foods.
Regarding the study by Malaveille et al. (2004) total and individual
polyphenols were not calculated because the intake of individual foods
was not provided.
The data for total and individual polyphenol intake from each study
were then integrated in order to obtain p25 (percentile 25), median, p75
(percentile 75), minimum and maximum values for polyphenol intake in
the studies selected. Unless expressly stated, all the results provided in
the Results section below correspond to median values.
3. Results
3.1. Search and study selection
The workow for paper selection is shown in Fig. 1A. While the
initial bibliography search of the Scopus and PUBMED databases pro-
vided a total of 1,756 publications, the application of the two inclusion
criteria to the titles and the exclusion of duplicated papers, resulted in
the identication of 33 publications for potential inclusion. From these,
reading the abstract and application of the six additional exclusion
criteria explained above nally left us with just 5 nutritional interven-
tion studies for our systematic review (Fig. 1A). The JADAD score of
each of these selected publications was 3 or 4 (Fig. 1B), reecting a high-
quality methodology.
3.2. Nutritional intervention studies
Characteristics of the selected nutritional studies are summarized in
Table 1. The interventions lasted from 8 to 12 weeks using mostly ran-
domized, controlled, parallel, single-blinded designs. The studies were
focused on several primary and secondary outcomes; four of the ve
studies selected found that the polyphenol-rich diet signicantly
improved the primary outcome established in each case.
Regarding subject characteristics, excepting one study (Malaveille
et al., 2004) where inclusion criteria did not consider cardiovascular risk
factors, ie., it was focused on male smokers of at least 15 cigarettes/day
for the last 10 years, the other ones considered some aspects related to
cardiovascular risk. Thus, they included both male and female subjects,
aged at least 30 and with a limit between 65 and 70 years old. And,
depending on the study, they should have some cardiovascular risk:
hypertension; overweight/obesity alone or combined with another fac-
tor of Metabolic Syndrome; cardiovascular risk according to Framing-
ham score
3.3. Polyphenol sources in the nutritional intervention studies
Once information on the diet in the selected studies was compiled, it
was possible to establish the food groups and individual food items
recommended to the volunteers in those studies. This information is
shown In Fig. 2. Fruit (100%), vegetables (80%), beverages (60%) and
cocoa products (60%) were the polyphenol sources in the intervention
studies included. Regarding individual foods, oranges (60%), blue-
berries (40%), strawberries (40%) and kiwis (40%) were the most
common fruit, while onion (80%), rocket (40%), spinach (40%), cab-
bage (40%) and tomato (40%) were selected as the richest polyphenol
sources in the vegetable group. In relation to the beverage intake,
decaffeinated green tea and coffee (40%) were the sources of poly-
phenol. Other specic food products were extra virgin olive oil (40%)
and dark chocolate (60%).
3.4. Intake of total polyphenols and main polyphenol classes in the
nutritional intervention studies
From the dietary recommendations given to the participants in the
studies included in our review, it was possible to establish the range of
total polyphenol intake as well as the intake of the different polyphenol
classes for each study, as shown in Fig. 3. Total polyphenol intakes
ranged from 11,394 (p25) to 27,060 (p75) mg/week, with a median
value of 17,945 (Fig. 3A), which we categorized into three polyphenol
groups according to the amount consumed: high, intermediate and low
(Fig. 3B-D). In the high group (Fig. 3B), hydroxycinnamic acids (4,659
mg/week) were the predominant polyphenol class, followed by avo-
nols (4,144 mg/week), avanols (1,593 mg/week), hydroxybenzoic
acids (880.1 mg/week), anthocyanins (239 mg/week), and proantho-
cyanidins (>100 mg/week). In the intermediate intake category, the
polyphenol classes present were avanones (45.3 mg/week), tyrosols
(35.3 mg/week) and avones (8.7 mg/week) (Fig. 3C). Finally, the
polyphenol classes with low intake were lignans (4.95 mg/week),
alkylmethoxyphenols (3.78 mg/week), stilbenes (0.725 mg/week), and
dihydrochalcones and isoavones (0.0 mg/week) (Fig. 3D). It should be
highlighted that most polyphenol classes showed considerable data
dispersion (for instance, for hydroxycinnamic acids, p25 was 301.1 mg/
week and p75 was 12,370 mg/week; and in avonols, these values were
353.1 and 7,288 mg/week), which may lead to different physiological
effects on individuals, all of whom were subjected to an overall high
polyphenol intake. In contrast, in some classes such as avanols, the
values were much more tightly grouped, with a p25 value of 853.3 mg/
week and a p75 value of 2,156 mg/week.
3.5. Intake of individual polyphenols in the nutritional intervention
studies
We obtained the mean intakes for individual polyphenols in the
different polyphenol classes in the studies included, and they are rep-
resented in Figs. 3-6. To facilitate clear data visualization, in categories
where the range of intakes for the different individual compounds were
very wide, they are represented as high, intermediate and low intake
(for instance, for hydroxycinnamic acids) or as high and low intake (as in
the case of anthocyanins).
3.5.1. Hydroxycinnamic acids
5-Caffeoylquinic acid was the hydroxycinnamic acid that was most
consumed in whole-diet intervention studies (1,742 mg/week), followed
by 3-caffeoylquinic acid (910.8 mg/week) and 4-caffeoylquinic acid
(846.2 mg/week) (Fig. 4A-1). The intermediate group of hydroxycin-
namic acid intake included 4-feruloylquinic acid (133.1 mg/week), 5-
feruloylquinic acid (125.8 mg/week), 4,5-dicaffeoylquinic acid (116.8
mg/week), ferulic acid (106.2 mg/week), 3,4-dicaffeoylquinic acid
(105.7 mg/week) and 3,5-dicaffeoylquinic acid (88.76 mg/week)
(Fig. 4A-2). Finally, three individual minor hydroxycinnamic acids were
found in the intervention studies: caffeoyl glucose (0.28 mg/week),
feruloyl glucose (0.28 mg/week) and cinnamic acid (0.17 mg/week)
(Fig. 4A-3).
3.5.2. Flavonols
Quercetin (2,418 mg/week) was the main avonol present in the
polyphenol-rich diets in the intervention studies (Fig. 4B-1). In the in-
termediate consumption group, myricetin (59.4 mg/week), quercetin 3-
O-rutinoside (40.9 mg/week), kaempferol (39.3 mg/week), iso-
rhamnetin (29.1 mg/week) and quercetin 3-O-galactoside (22.2 mg/
week) were found in the dietary intervention studies (Fig. 4B-2). Minor
L. Condezo-Hoyos et al.

Food Research International 149 (2021) 110655
4
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) owchart of study inclusion (A); and JADAD scores of the selected publi-
cations according to key methodological features (B).
L. Condezo-Hoyos et al.

Food Research International 149 (2021) 110655
5
avonols included kaempferol 3-O-galactoside (11.8 mg/week), patu-
letin 3-O-(2
′
’-feruloylglucosyl)(1->6)-[apiosyl(1->2)]-glucoside (7.3
mg/week), quercetin 3-O-glucuronide (4.5 mg/week) and spinacetin 3-
O-glucosyl-(1->6)-glucoside (3.5 mg/week) (Fig. 4B-3).
3.5.3. Flavanols
(-)-Epigallocatechin 3-O-gallate (380.2 mg/week), (-)-epi-
gallocatechin (275.7 mg/week), (-)-epicatechin (151.3 mg/week),
(-)-epicatechin 3-O-gallate (106.6 mg/week) and cinnamtannin A2
(94.2 mg/week) were the avanols with the highest intake in the dietary
interventional studies (Fig. 4C-1). While procyanidin dimer B2 (84.9
mg/week), procyanidin trimer C1 (75.5 mg/week), (+)-catechin (55.5
mg/week), (+)-gallocatechin (31.8 mg/week), and procyanidin dimer
B4 (26.9 mg/week) were identied in the intermediate intake group
(Fig. 4C-2). Finally, procyanidin dimers B1 (15.7 mg/week) and B7
(13.2 mg/week) were the minor avanols in the nutritional intervention
studies (Fig. 4C-3).
Table 1
General characteristics of the nutritional intervention studies included in this systematic review.
Diet design Study design References
Serving size Degree of detail Strategy for assessing
dietary adherence
Study type Subject characteristics Primary
outcome
Secondary
outcomes
NS Meals were prepared
by a qualied
catering service
under the supervision
of the team of
dietitians
Every week participants
were given meals and
beverages, for the
whole duration of
intervention, in
amounts sufcient to
cover overall household
needs
Randomized,
controlled,
parallel, single-
blinded
Men and women aged
between 35 and 70 years
with overweight or
obesity (BMI 27–35),
high waist
circumference (above
102 cm for men or 88 cm
for women), and at least
one more feature of the
metabolic syndrome
based on the National
Cholesterol Education
Program/Adult
Treatment Program
Postprandial
lipid response*
Glucose, insulin,
GLP-1,
microbiota,
oxidative stress
Della et al.
(2020)
Vetrani et al.
(2020)
Vetrani et al.
(2018)
Bozzetto et al.
(2015)
Based on UK Food
Standards
Agency
guidelines
6 daily portions of
F&V including one
portion of berries and
50 g of chocolate
(70% cocoa) , per
day.
The high-polyphenol
diet had a self-selected
weekly delivery of the
diet, free of charge, to
participants’ homes
from a local
supermarket. Each
participant was also
contacted by telephone
at weekly intervals
Randomized,
controlled,
parallel, single-
blinded
Men and women aged
40–65 years, with
documented grade I
(140–159/90–99 mm
Hg) or grade II
(160–179/100–109 mm
Hg) hypertension.
Forearm
blood ow
responses to an
endothelium-
dependent
vasodilator*
Blood pressure,
lipid prole,
body mass index
Noad et al.
(2016)
Green tea 400 ml,
dark chocolate
25 g, blueberry
jam 50 g,
artichokes 300 g,
onions 200 g,
spinach 150 g
and rocket 90 g
Meals were prepared
by a qualied
catering service
under the supervision
of the team of
dietitians
To improve diet
adherence, meals and
beverages were
provided to the
participants for the
whole study period in
amounts sufcient to
cover their household
consumption
Randomized,
controlled,
parallel, single-
blinded
Men and women aged
between 35 and 70 years
with overweight or
obesity (BMI 27–35),
high waist
circumference (above
102 cm for men or 88 cm
for women)
Postprandial
triglycerides*
Fasting lipid
prole, urinary
isoprostanes
Annuzzi et al.
(2014)
2 portions per day
additionally
every 6 weeks of
the study
reaching a
maximum of 6
extra portions
per day by week
18
Participants were
encouraged to
consume a variety of
F&V or composite
foods from the list
each week and have
equal proportions of
F&V
Participants in the
intervention groups
indicated the type and
number of additional
portions of F&V they
consumed each day on
record forms. A
minimum of two
random structured 24 h
dietary recalls
Randomized,
controlled,
parallel, single-
blinded
Male and women aged
30–70 years.
Framingham risk score
system was used for
recruitment based on
scoring a minimum of 2
points in one or more of
the following criteria:
(1) total plasma
cholesterol, (2) high-
density lipoprotein
(HDL) cholesterol, (3)
blood pressure, (4)
smoking status; (5)
obesity/adiposity and
(6) body mass index
(BMI)
Vascular
reactivity*
Plasma and
urine vitamin C,
carotenoids,
avonoids, uric
acid, antioxidant
capacity
Chong et al.
(2013);
McReady
et al. (2014)
NS The dietician
developed a food-
nutrient-intake
matrix specically
focused on
avonoids, to
quantitatively assess
the intake
All participants lled in
a food-frequency
questionnaire (FFQ) at
the beginning of the
study, and then lled in
a daily dietary diary
during the experimental
month
Randomized,
controlled,
parallel, single-
blinded
Male smokers of at least
15 cigarettes/day for the
last 10 years
Urine DNA
adducts
Urine phenolic
compounds
Malaveille
et al. (2004)
NS, non-specied.
*
Signicant differences (p > 0.05) were observed between low- and high- polyphenol diet, showing benecial effects of high-polyphenol diet.
L. Condezo-Hoyos et al.

Food Research International 149 (2021) 110655
6
Fig. 2. Food groups and individual foods used as polyphenol sources in the nutritional intervention studies included in this systematic review.
Fig. 3. Intake of total polyphenols (A), and main polyphenol classes (B-D), calculated from diets used in the nutritional intervention studies included in this sys-
tematic review. Polyphenol contents were obtained from Phenol-Explore and USDA databases, and from some specic research papers for foods missing from those
databases. The values are expressed as mg/week.
L. Condezo-Hoyos et al.

Food Research International 149 (2021) 110655
7
Fig. 4. Intake of individual hydroxycinnamic acids, avonols and avanols in the nutritional intervention studies included in this systematic review. For polyphenol
class, individual compounds were grouped as high, intermediate or low intake. The values are expressed as mg of individual polyphenol/week.
Fig. 5. Intake of individual anthocyanins in the nutritional intervention studies included in this systematic review. Individual compounds were grouped as high or
low intake. The values are expressed as mg of individual anthocyanin/week.
L. Condezo-Hoyos et al.
Food Research International 149 (2021) 110655
8
3.5.4. Anthocyanins
Forty-seven individual anthocyanins were found in the polyphenol-
rich diets of the intervention studies (Fig. 5). We divided them into
two categories. The rst included those with a mean intake higher than
5 mg/week: pelargonidin 3-O-glucoside (37.2 mg/week), cyanidin 3-O-
galactoside (11 mg/week), pelargonidin 3-O-(6
′
’-succinyl-glucoside)
(8.0 mg/week), cyanidin-3-O-glucoside (10.8 mg/week), cyanidin 3-O-
glucosyl-rutinoside (8.1 mg/week), cyanidin 3-O-(6
′
’-malonyl-3
′
’-glu-
cosyl-glucoside) (6.6 mg/week), cyanidin 3-O-arabinoside (6.2 mg/
week) and cyanidin 3-O-(6
′
’-acetyl-glucoside) (4.7 mg/week). The other
anthocyanins had an intake of less than 5 mg/week. However, some
anthocyanins in the rst category, showed extremely dispersed data: for
cyanidin 3-O-rutinoside and cyanidin 3-O-glucoside, intake data ranged
from 0 to 300 mg/week.
3.5.5. Hydroxybenzoic acids
In the case of hydroxybenzoic acids, 2-hydroxybenzoic (377.6 mg/
week) was the most consumed in the dietary intervention studies
(Fig. 6A-1). 5-O-Galloylquinic acid (131.8 mg/week), gallic acid (97.8
mg/week), sanguiin H-6 (29.0 mg/week), ellagic acid (19.68 mg/week)
and lambertianin C (11.8 mg/week) were also among the most
consumed hydroxybenzoic acids (Fig. 6A-1). As minor hydroxybenzoic
acids, the study diets also contained ellagic acid glucoside (2.2 mg/
week) and 4-hydroxybenzoic acid 4-O-glucoside (2 mg/week) (Fig. 6A-
2).
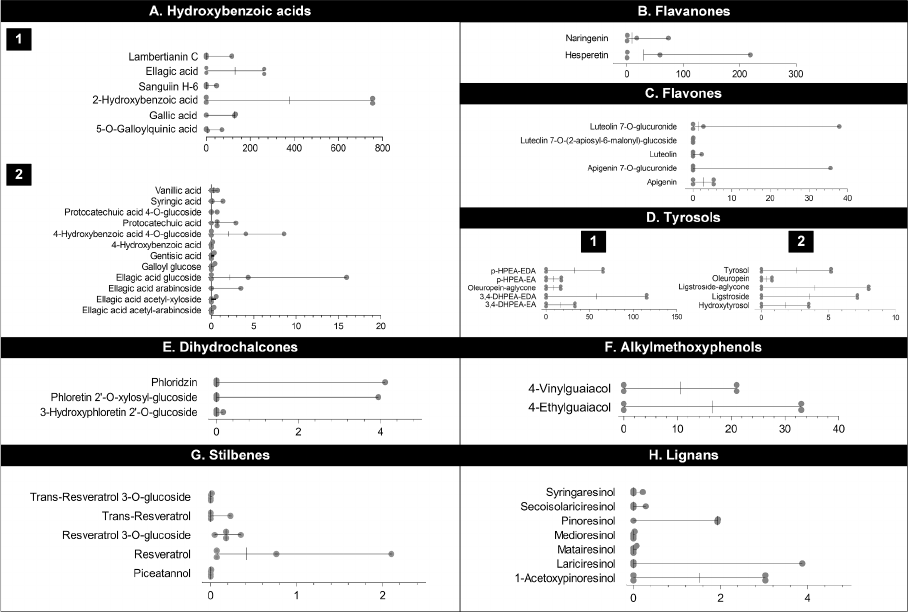
3.5.6. Tyrosols, avanones and alkylmethoxyphenols
The predominant tyrosols found in the polyphenol-rich diets in the
studies were 3,4-DHPEA-EDA (57.7 mg/week), p-HPEA-EDA (32.7 mg/
week), 3,4-DHPEA-EA (16.6 mg/week), p-HPEA-EA (8.7 mg/week) and
oleuropein-aglycone (8.4 mg/week). Minor tyrosols included ligstroside
(3.6 mg/week) and ligstroside-aglycone (4.0 mg/week) (Fig. 6D). In
addition, the diets provided hesperetin (29.4 mg/week) and naringenin
(8.9 mg/week) as avanones (Fig. 6B). The only alkylmethoxyphenols
provided by the diets were 4-ethylguaiacol (6.5 mg/week) and 4-vinyl-
guaiacol (10.5 mg/week) (Fig. 6F).
3.5.7. Other minor individual polyphenols
Flavones such as apigenin (2.7 mg/week) and luteolin 7-O-glucuro-
nide (1.4 mg/week), the stilbenes resveratrol (0.4 mg/week) and
resveratrol 3-O-glucoside (0.2 mg/week), and the lignans pinoresinol
(1.9 mg/week) and 1-acetoxypinoresinol (1.5 mg/week) were all found
in the diet intervention studies (Fig. 6C, 6G and 6H).
4. Discussion
The exploration of dietary patterns as a whole is increasingly pro-
moted in the eld of nutrition, and it may be applied to the topic of
polyphenol research. However, the characteristics that a diet should
have in order to be considered as genuinely rich in polyphenols have not
been explored or discussed, despite this being a key aspect in order to be
able to compare the efciency of nutritional interventions. In the present
systematic review, we aimed to explore the specic characteristics of the
diets used in nutritional interventions focused on polyphenol-rich diets,
in order to arrive at some general recommendations for future research.
The JADAD score is considered the most valid and reliable tool to assess
the methodological quality of a clinical trial, and has been applied
throughout nutritional studies (Bisol et al., 2020; Neelakantan et al.,
2020; Roman et al., 2018). Nonetheless, some authors have identied
limitations of this system, such as not including a point for blinding
during outcome assessment, only for single blinding (McCormick et al.,
2013).
Fig. 6. Intake of individual avanols, avanones, avones, tyrosols, dihydrochalcones, alkylmethoxyphenols, stilbenes and lignans in the nutritional intervention
studies included in this systematic review. In each group of polyphenols, individual compounds were grouped as high, intermediate or low intake. The values are
expressed as mg of individual polyphenol/week.
L. Condezo-Hoyos et al.
Food Research International 149 (2021) 110655
9
The rst relevant observation of this study is that clinical trials
assessing the effect of a polyphenol-rich diet as a whole are scarce, since
most interventions involving polyphenols focus on extracts or individual
foods, in order to reduce the interferences from the multiple variables
present in studies on whole diets. Assessment of individual foods is
clearly a necessary stage in the evaluation of the health effects of
polyphenols, but it should be followed by studies of whole diets, since in
a real situation these are what subjects consume. Thus, we consider ef-
forts should be performed for increasing studies evaluating polyphenol-
rich intake within a whole dietary pattern. The studies included in this
systematic review evaluated several primary and secondary clinical
outcomes, but we should emphasize that this systematic review focuses
on the design of a polyphenol-rich diet itself. Also, there were differ-
ences among the studied subjects; although some dietary recommen-
dations for polyphenol intake may be general for any population, in case
some specic recommendation was provided due to the characteristics
of the subjects (for instance, adapting isoavone intake to menopausal
situation), this should be reported.
The clinical trials included in this systematic review showed, rst of
all, considerable variability in terms of the instructions provided to the
volunteers. They varied from studies where participants had to consume
a particular food combination every day (Annuzzi et al., 2014) to others
where they were instructed to consume a daily number of servings of
polyphenol-rich foods which they selected themselves (Chong et al.,
2013), or still others where the participants received pre-prepared meals
(Della Pepa et al., 2020). Regarding the specic food classes or items
recommended, although all the studied included fruit, not all recom-
mended vegetables, cocoa products or beverages as polyphenol sources;
similarly, some foods with rather characteristic polyphenol content,
such as olive oil or green tea, were not included in the food selection of
all the studies. This generates an initial problem when comparing the
trials. At the same time, although the studies suggesting or providing
volunteers with a xed combination of foods generated a more ho-
mogenous intervention and therefore, potentially, biological responses,
those where the volunteers are requested to perform their food selection
are closer to a real situation, where food combinations contribute to
polyphenol intake and, ultimately, to their associated health effects (de
Pascual-Teresa & Clifford, 2017).
A notable aspect is that, although the clinical trials included were
focused on polyphenol-rich diets, sometimes they did not include an
evaluation of the polyphenol intake of the subjects (either total or by
individual compounds), which we therefore calculated specically for
this study using existing databases. Thus, one recommendation would be
that, in such studies as these, an initial estimation of the polyphenol
intake of the volunteers should be made, in order to know exactly how
far or close to usual polyphenol intake the diet provided will be in that
population or in similar ones. Overall, the median polyphenol intake of
the volunteers in the clinical trials was about 2 g/day, which corre-
sponds to a high polyphenol intake, compared to those reported in
several populations (Nascimento-Souza et al., 2018; P
´
erez-Jim
´
enez
et al., 2011; Tresserra-Rimbau et al., 2013), but within the range that
may be achieved by a standard diet, without supplementation. Despite
this general characteristic of a high polyphenol intake, which is in
agreement with the purposes of the studies included, considerable
variation in polyphenol intake was observed between the studies, with a
more than two-fold difference between p25 and p75 values. This
affected most polyphenol classes, (for instance, in hydroxycinnamic
acids, weekly intake p75 values were up to ten-fold higher than p25
values) and also individual polyphenols, although in some classes (e.g.,
avanols) the intake range was very similar between the studies. In
general, these substantial differences in the polyphenol intake of the
volunteers make it rather difcult to compare them between the clinical
trials, and this may contribute to explaining some of the discrepancies
observed in the results. Nevertheless, most of the studies evaluating
biochemical outcomes found benecial effects derived from a
polyphenol-rich diet, independently on whether they were in the low
(Noad et al., 2016) or the high (Annuzzi et al., 2014; Della Pepa et al.,
2020) polyphenol intake range. (Although one of the studies did not nd
any effect on the formation of urinary DNA adducts (Malaveille et al.,
2014) but, as stated below, the information provided did not allow us to
calculate individual polyphenol intake in this case.) This was due to the
fact that, despite the differences in polyphenol intake between the
specic studies, they all corresponded to a high polyphenol intake, so
this is in agreement with the reported biological effects associated with
this condition. To be more precise, the studies with the lowest weekly
polyphenol intake (10 g/week) provided a polyphenol dose which was
clearly higher than that observed for the quartile with the lowest poly-
phenol intake in a Brazilian population, which was about 4 g/week
(Nascimento-Souza et al., 2018).
At the same time, we cannot ignore that the estimation of polyphenol
intake from databases is subject to all the well-known drawbacks that
those databases still possess, due to analytical limitations such as the
problem for properly estimating proanthocyanidin content (P
´
erez-
Jim
´
enez et al., 2010) or the lack of inclusion of non-extractable poly-
phenols, despite their contribution to total polyphenol intake (P
´
erez-
Jim
´
enez & Saura-Calixto, 2015). This is combined with the potential
lack of or partial compliance on the part of participants, which means
that determination of polyphenol metabolites in biological uids is
highly recommended in this kind of study, as they can be used as bio-
markers of intake according to robust procedures (Dragsted et al., 2018),
and to establish associations between circulating doses and associated
health outcomes, especially as the metabolic fate of polyphenols is
highly affected by inter-individual variability (Morand & Tom
´
as-Bar-
ber
´
an, 2019).
A general reection arises from this systematic review. Currently,
there is a tendency to study dietary patterns as a whole. This includes,
for instance, the widely studied Mediterranean diet, but also dietary
patterns focused on both health and sustainability (Willett et al., 2019).
In this context, some intervention studies have explored the role of
polyphenol-rich diets as a whole; especially because, as recently
reviewed, there is evidence of an association between polyphenol-rich
dietary patterns assessed in observational studies and different health
outcomes (Del Bo’ et al., 2019). However, the present systematic review
shows that the amounts of polyphenols provided in these clinical trials
may differ widely. This situation is similar to other aspects of polyphenol
research, where differences in study design (Nú
˜
nez-S
´
anchez et al., 2015;
Mariano et al., 2020), analytical determinations (Pokimica & García-
Coonesa, 2018), data reporting (Nikolic et al., 2019) or nomenclature
(Kay et al., 2020) have been observed by researchers and have led to
joint recommendations for homogenization. Similarly, a joined effort by
polyphenol researchers community could be made to establish certain
parameters for a polyphenol-rich diet. This does not mean a single,
universal model (clearly, it makes no sense to recommend daily con-
sumption of tropical fruit in northern countries, for example). However,
in the same way that different indexes have been established for the
Mediterranean dietary pattern which does not mean that the diet is the
same in Italy and Turkey, some general concepts about a polyphenol-
rich dietary pattern could be established. These could include ranges
for total polyphenol intake, polyphenol classes and certain individual
polyphenols (based on current knowledge, from both observational and
intervention studies), which could then be adapted to local food con-
sumption patterns. Indeed, it has been observed that some current di-
etary guidelines already promote polyphenol-rich dietary patterns
(Castro-Acosta et al., 2019) so they would not require substantial
modications. And epidemiological studies have shown that it is
possible to reach a similar polyphenol intake based on different char-
acteristic local products, such as black beans in Brazil (Nascimento
–Souza et al., 2018) or olives and olive oil in Spain (Tresserra-Rimbau
et al., 2013). Of course, at the same time, studies focused on individual
compounds or foods are clearly needed in order to advance our
knowledge of the health effects of polyphenols, for which information is
still limited in many aspects.
L. Condezo-Hoyos et al.
Food Research International 149 (2021) 110655
10
This systematic review is the rst simultaneous comparison of
intervention studies based on the concept of a polyphenol-rich diet, and
it raises some aspects not previously observed, such as the differences in
intake, in particular for some polyphenol classes. This study also has
some limitations though, such as the small number of trials identied
that full our inclusion and exclusion criteria; the differences in the
populations studied; the different approaches for estimating polyphenol
intake in the studies; and the different biases that affect all intervention
studies, particularly nutritional ones. At the same time, it obtained some
useful take-away ndings: more intervention studies should explore the
effects of polyphenol-rich diet; such studies should provide information
as detailed as possible on the dietary recommendations provided to the
subjects (products, amount, cooking conditions, food origin, etc.); in-
formation on expected individual polyphenol intake by study subjects
and how it was determined should also be provided; in case specic
polyphenol intake recommendations were done due to the characteris-
tics of the population, this should be stated.
5. Conclusions
The number of clinical trials that assess the effect of polyphenol-rich
diets on health biomarkers is still limited. Moreover, such studies show
important differences in terms of the details provided to the volunteers
or the foods included, and therefore the total and individual polyphenol
intake. This may affect the results observed and make the comparison of
different nutritional interventions difcult. Due to the evidence on the
health-related properties of polyphenols and the need to develop holistic
approaches to dietary patterns, some general characteristics of a
polyphenol-rich diet may deserve further exploration.
CRediT author contribution statement
J. P
´
erez-Jim
´
enez conceived the study and supervised all the tasks; C.
Gazi and L. Condezo-Hoyos performed the bibliography search; L.C.-H.
carried out data curation and visualization; J.P.-J. and L.C.-H. inter-
preted the results and wrote the rst draft of the manuscript. This nal
version of the manuscript has been reviewed and approved by all the
authors.
Declaration of Competing Interest
The authors declare that they have no known competing nancial
interests or personal relationships that could have appeared to inuence
the work reported in this paper.
Acknowledgments
Miss Christina Gazi was the recipient of an Erasmus+grant, a pro-
gramme from the European Union Language revision by Christopher
Evans is acknowledged.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.
org/10.1016/j.foodres.2021.110655.
References
Akaberi, M., & Hosseinzadeh, H. (2016). Grapes (Vitis vinifera) as a Potential Candidate
for the Therapy of the Metabolic Syndrome. Phytotherapy Research : PTR, 30. https://
doi.org/10.1002/ptr.5570.
Annuzzi, G., Bozzetto, L., Costabile, G., Giacco, R., Mangione, A., Anniballi, G.,
Vitale, M., Vetrani, C., Cipriano, P., Della Corte, G., Pasanisi, F., Riccardi, G., &
Rivellese, A. A. (2014). Diets naturally rich in polyphenols improve fasting and
postprandial dyslipidemia and reduce oxidative stress: A randomized controlled
trial. American Journal of Clinical Nutrition. https://doi.org/10.3945/
ajcn.113.073445.
Bhagwat, S., & Haytowitz, D. B. (2007). USDA Database for the Flavonoid Contents of
Selected Foods. Release, 08.
Bisol,
ˆ
A., de Campos, P. S., & Lamers, M. L. (2020). Flavonoids as anticancer therapies: A
systematic review of clinical trials. Phytotherapy Research. https://doi.org/10.1002/
ptr.6551.
Bondonno, N. P., Dalgaard, F., Kyrø, C., Murray, K., Bondonno, C. P., Lewis, J. R.,
Croft, K. D., Gislason, G., Scalbert, A., Cassidy, A., Tjønneland, A., Overvad, K., &
Hodgson, J. M. (2019). Flavonoid intake is associated with lower mortality in the
Danish Diet Cancer and Health Cohort. Nature Communications. https://doi.org/
10.1038/s41467-019-11622-x.
Bozzetto, L., Annuzzi, G., Pacini, G., Costabile, G., Vetrani, C., Vitale, M., Griffo, E.,
Giacco, A., De Natale, C., Cocozza, S., Della Pepa, G., Tura, A., Riccardi, G., &
Rivellese, A. A. (2015). Polyphenol-rich diets improve glucose metabolism in people
at high cardiometabolic risk: A controlled randomised intervention trial.
Diabetologia, 58(7), 1551–1560. https://doi.org/10.1007/s00125-015-3592-x.
Castro-Acosta, M. L., Sanders, T. A. B., Reidlinger, D. P., Darzi, J., & Hall, W. L. (2019).
Adherence to UK dietary guidelines is associated with higher dietary intake of total
and specic polyphenols compared with a traditional UK diet: Further analysis of
data from the Cardiovascular risk REduction Study: Supported by an Integrated
Dietary Approach (CRESSIDA) randomised controlled trial. The British Journal of
Nutrition, 121(4), 402–415. https://doi.org/10.1017/S0007114518003409.
Chong, M. F., George, T. W., Alimbetov, D., Jin, Y., Weech, M., MacReady, A. L.,
Spencer, J. P. E., Kennedy, O. B., Minihane, A. M., Gordon, M. H., & Lovegrove, J. A.
(2013). Impact of the quantity and avonoid content of fruits and vegetables on
markers of intake in adults with an increased risk of cardiovascular disease: The
FLAVURS trial. European Journal of Nutrition, 52(1), 361–378. https://doi.org/
10.1007/s00394-012-0343-3.
de Pascual-Teresa, S., & Clifford, M. N. (2017). Advances in Polyphenol Research: A
Journal of Agricultural and Food Chemistry Virtual Issue. Journal of Agricultural and
Food Chemistry, 65(37), 8093–8095. https://doi.org/10.1021/acs.jafc.7b04055.
Del Bo’, C., Bernardi, S., Marino, M., Porrini, M., Tucci, M., Guglielmetti, S., Cherubini,
A., Carrieri, B., Kirkup, B., Kroon, P., Zamora-Ros, R., Liberona, N. H., Andres-
Lacueva, C., & Riso, P. (2019). Systematic Review on Polyphenol Intake and Health
Outcomes: Is there Sufcient Evidence to Dene a Health-Promoting Polyphenol-
Rich Dietary Pattern? Nutrients, 11(6). https://doi.org/10.3390/nu11061355.
Della Pepa, G., Vetrani, C., Vitale, M., Bozzetto, L., Costabile, G., Cipriano, P.,
Mangione, A., Patti, L., Riccardi, G., Rivellese, A. A., & Annuzzi, G. (2020). Effects of
a diet naturally rich in polyphenols on lipid composition of postprandial lipoproteins
in high cardiometabolic risk individuals: An ancillary analysis of a randomized
controlled trial. European Journal of Clinical Nutrition. https://doi.org/10.1038/
s41430-019-0459-0.
Dragsted, L. O., Gao, Q., Scalbert, A., Verg
`
eres, G., Kolehmainen, M., Manach, C.,
Brennan, L., Afman, L. A., Wishart, D. S., Andres Lacueva, C., Garcia-Aloy, M.,
Verhagen, H., Feskens, E. J. M., & Pratic
`
o, G. (2018). Validation of biomarkers of
food intake-Critical assessment of candidate biomarkers. Genes and Nutrition, 13(1),
1–14. https://doi.org/10.1186/s12263-018-0603-9.
Giallourou, N., Oruna-Concha, M. J., & Harbourne, N. (2016). Effects of domestic
processing methods on the phytochemical content of watercress (Nasturtium
ofcinale). Food Chemistry, 212, 411–419. https://doi.org/10.1016/j.
foodchem.2016.05.190.
Gianfredi, V., Salvatori, T., Nucci, D., Villarini, M., & Moretti, M. (2018). Can chocolate
consumption reduce cardio-cerebrovascular risk? A systematic review and meta-
analysis. Nutrition (Burbank, Los Angeles County, Calif.), 46, 103–114. https://doi.
org/10.1016/j.nut.2017.09.006.
Heshmati, J., Golab, F., Morvaridzadeh, M., Potter, E., Akbari-Fakhrabadi, M., Farsi, F.,
Tanbakooei, S., & Shidfar, F. (2020). The effects of curcumin supplementation on
oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ
coactivator 1
α
gene expression in polycystic ovarian syndrome (PCOS) patients: A
randomized placebo-controlled clinical trial. Diabetes & Metabolic Syndrome: Clinical
Research and Reviews, 14, 77–82. https://doi.org/10.1016/j.dsx.2020.01.002.
Je
˙
z, M., Wiczkowski, W., Zieli
´
nska, D., Białobrzewski, I., & Błaszczak, W. (2018). The
impact of high pressure processing on the phenolic prole, hydrophilic antioxidant
and reducing capacity of pur
´
ee obtained from commercial tomato varieties. Food
Chemistry, 261, 201–209. https://doi.org/10.1016/j.foodchem.2018.04.060.
Kay, C. D., Clifford, M. N., Mena, P., McDougall, G. J., Andr
´
es-Lacueva, C., Cassidy, A.,
Del Rio, D., Kuhnert, N., Manach, C., Pereira-Caro, G., Rodríguez-Mateos, A.,
Scalbert, A., Tom
´
as-Barber
´
an, F. A., Williamson, G., Wishart, D. S., & Crozier, A.
(2020). Recommendations for standardizing nomenclature for dietary (poly)phenol
catabolites. American Journal of Clinical Nutrition, 112, 1051–1068. https://doi.org/
10.1093/ajcn/nqaa204.
Malaveille, C., Fiorini, L., Bianchini, M., Davico, L., Bertinetti, S., Allegro, G.,
Hautefeuille, A., Sacerdote, C., & Vineis, P. (2004). Randomized controlled trial of
dietary intervention: Association between level of urinary phenolics and anti-
mutagenicity. Mutation Research - Genetic Toxicology and Environmental Mutagenesis.
https://doi.org/10.1016/j.mrgentox.2004.03.007.
Marino, M., Del Bo, C., Martini, D., Porrini, M., & Riso, P. (2020). A review of registered
clinical trials on dietary (poly)phenols: Past efforts and possible future directions.
Foods, 9, 1606–1621. https://doi.org/10.3390/foods9111606.
Martini, D., Chaiavaroli, L., Gonz
´
alez-Sarrías, A., Bresciani, L., Palma-Dur
´
an, S.,
Dall’Asta, M., Deligiannidou, G. E., Massaro, M., Scoditti, E., Combet, E.,
Maskimova, V., Urpi-Sarda, M., Kontogiorgis, C. A., Andr
´
es-Lacueva, C.,
Gibney, E. R., Del Rio, D., Morand, C., García-Aloy, M., Rodríguez-Mateos, A., &
Mena, P. (2019). Impact of foods and dietary supplements containing
hydroxycinnamic acids on cardiometabolic biomarkers: A systematic review to
explore inter-individual variability. Nutrients, 5–34. https://doi.org/10.3390/
nu11081805.
McCormick, F., Cvetanovich, G. L., Kim, J. M., Harris, J. D., Gupta, A. K., Abrams, G. D.,
Romeo, A. A., & Provencher, M. T. (2013). An assessment of the quality of rotator
L. Condezo-Hoyos et al.
Food Research International 149 (2021) 110655
11
cuff randomized controlled trials: Utilizing the Jadad score and CONSORT criteria.
Journal of Shoulder and Elbow Surgery, 22(9), 1180–1185. https://doi.org/10.1016/j.
jse.2013.01.017.
McReady, George, T.W., Chong, M.F., Alimbetov, D.S., Jin, Y., Vidal, A., Spencer, J.P.E.,
Kennedy, O.B., Tuohy, K.M., Minihane, A.M., Gordon, M.H., Lovegrove, J. A. (2014)
Flavonoid-rich fruit and vegetables improve microvascular reactivity and
inammatory status in men at risk of cardiovascular disease-FLAVURS: A
randomized controlled trial. American Journal of Clinical Nutrition, 99, 479-89.
https://doi.org/ 10.3945/ajcn.113.074237.
Molinar-Toribio, E., Fuguet, E., Ramos-Romero, S., Taltavull, N., M
´
endez, L.,
Nogu
´
es, M. R., Medina, I., Torres, J. L., & P
´
erez-Jim
´
enez, J. (2018). A high-fat high-
sucrose diet affects the long-term metabolic fate of grape proanthocyanidins in rats.
European Journal of Nutrition. https://doi.org/10.1007/s00394-016-1323-9.
Morand, C., & Tom
´
as-Barber
´
an, F. A. (2019). Interindividual Variability in Absorption,
Distribution, Metabolism, and Excretion of Food Phytochemicals Should Be
Reported. Journal of Agricultural and Food Chemistry, 67(14), 3843–3844. https://doi.
org/10.1021/acs.jafc.9b01175.
Morvaridzadeh, M., Sepidarkish, M., Daneshzad, E., Akbari, A., Mobini, G.R., Heshmati,
J. (2020A) The effect of pomegranate on oxidative stress parameters: A systematic
review and meta-analysis. Complementary Therapies in Medicine, 48, Article ID
102252, https://doi.org/10.1016/j.ctim.2019.102252.
Morvaridzadeh, M., Fazelian, S., Agah, S., Khazdouz, M., Rahimlou, M., Agh, F., Potter,
E., Heshmati, S. & Heshmati, J. (2020) Effect of ginger (Zingiber ofcinale) on
inammatory markers: A systematic review and meta-analysis of randomized
controlled trials. Cytokine, 135, Article ID 155224, https://doi.org/10.1016/j.
cyto.2020.155224.
Nascimento-Souza, M. A., de Paiva, P. G., P
´
erez-Jim
´
enez, J., do Carmo Castro
Franceschini, S., & Ribeiro, A. Q. (2018). Estimated dietary intake and major food
sources of polyphenols in elderly of Viçosa, Brazil: A population-based study.
European Journal of Nutrition, 57(2), 617–627. https://doi.org/10.1007/s00394-
016-1348-0.
Neelakantan, N., Seah, J. Y. H., & van Dam, R. M. (2020). The Effect of Coconut Oil
Consumption on Cardiovascular Risk Factors: A Systematic Review and Meta-
Analysis of Clinical Trials. Circulation, 141(10), 803–814. https://doi.org/10.1161/
CIRCULATIONAHA.119.043052.
Neveu, V., Perez-Jim
´
enez, J., Vos, F., Crespy, V., du Chaffaut, L., Mennen, L., Knox, C.,
Eisner, R., Cruz, J., Wishart, D., & Scalbert, A. (2010). Phenol-Explorer: An online
comprehensive database on polyphenol contents in foods. Database: The Journal of
Biological Databases and Curation, 2010. https://doi.org/10.1093/database/
bap024.
Nikolic, M., Konic Ristic, A., Gonz
´
alez-Sarrías, A., Istas, G., Urpi-Sarda, M., Dall’Asta, M.,
Monfoulet, L.-E., Cloetens, L., Bayram, B., Tumolo, M. R., Chervenkov, M., Scoditti,
E., Massaro, M., Tejera, N., Abadjieva, D., Chambers, K., Krga, I., Tom
´
as-Barber
´
an, F.
A., Morand, C., … Mena, P. (2019). Improving the reporting quality of intervention
trials addressing the inter-individual variability in response to the consumption of
plant bioactives: Quality index and recommendations. European Journal of
Nutrition, 58(Suppl 2), 49–64. https://doi.org/10.1007/s00394-019-02069-3.
Noad, R. L., Rooney, C., McCall, D., Young, I. S., McCance, D., McKinley, M. C.,
Woodside, J. V., & McKeown, P. P. (2016). Benecial effect of a polyphenol-rich diet
on cardiovascular risk: A randomised control trial. Heart. https://doi.org/10.1136/
heartjnl-2015-309218.
Nú
˜
nez-S
´
anchez, M. A., Gonz
´
alez-Sarrías, A., Romo-Vaquero, M., García-Villalba, R.,
Selma, M. V., Tom
´
as-Barber
´
an, F. A., García-Conesa, M.-T., & Espín, J. C. (2015).
Dietary phenolics against colorectal cancer–From promising preclinical results to
poor translation into clinical trials: Pitfalls and future needs. Molecular Nutrition &
Food Research, 59(7), 1274–1291. https://doi.org/10.1002/mnfr.201400866.
P
´
erez-Jim
´
enez, J., Fezeu, L., Touvier, M., Arnault, N., Manach, C., Hercberg, S.,
Galan, P., & Scalbert, A. (2011). Dietary intake of 337 polyphenols in French adults.
The American Journal of Clinical Nutrition, 93(6), 1220–1228. https://doi.org/
10.3945/ajcn.110.007096.
P
´
erez-Jim
´
enez, J., Neveu, V., Vos, F., & Scalbert, A. (2010). Systematic analysis of the
content of 502 polyphenols in 452 foods and beverages: An application of the
phenol-explorer database. Journal of Agricultural and Food Chemistry, 58(8),
4959–4969. https://doi.org/10.1021/jf100128b.
P
´
erez-Jim
´
enez, J., & Saura-Calixto, F. (2015). Macromolecular antioxidants or non-
extractable polyphenols in fruit and vegetables: Intake in four European countries.
Food Research International, 74, 315–323. https://doi.org/10.1016/j.
foodres.2015.05.007.
Pokimica, B., & García-Conesa, M. T. (2018). Critical evaluation of gene expression
changes in human tissues in response to supplementation with dietary bioactive
compounds: Moving towards better-quality studies. Nutrients, 10, 807–844. https://
doi.org/10.3390/nu10070807.
Reis, C. E. G., D
´
orea, J. G., & da Costa, T. H. M. (2019). Effects of coffee consumption on
glucose metabolism: A systematic review of clinical trials. Journal of Traditional and
Complementary Medicine, 9(3), 184–191. https://doi.org/10.1016/j.
jtcme.2018.01.001.
Rodriguez-Mateos, A., Vauzour, D., Krueger, C. G., Shanmuganayagam, D., Reed, J.,
Calani, L., Mena, P., Del Rio, D., & Crozier, A. (2014). Bioavailability, bioactivity and
impact on health of dietary avonoids and related compounds: An update. Archives
of Toxicology, 88(10), 1803–1853. https://doi.org/10.1007/s00204-014-1330-7.
Roman, P., Carrillo-Trabal
´
on, F., S
´
anchez-Labraca, N., Ca
˜
nadas, F., Est
´
evez, A. F., &
Cardona, D. (2018). Are probiotic treatments useful on bromyalgia syndrome or
chronic fatigue syndrome patients? A systematic review. Benecial Microbes, 9(4),
603–611. https://doi.org/10.3920/BM2017.0125.
Santos, J., Oliveira, M. B. P. P., Ib
´
a
˜
nez, E., & Herrero, M. (2014). Phenolic prole
evolution of different ready-to-eat baby-leaf vegetables during storage. Journal of
Chromatography. A, 1327, 118–131. https://doi.org/10.1016/j.chroma.2013.12.085.
´
Scibisz, I., & Mitek, M. (2007). The changes of antioxidant properties in highbush
blueberries (Vaccinium corymbosum L.) during freezing and long-term frozen
storage. Acta Scientiarum Polonorum Technologia. Alimentaria, 6(4). https://www.
food.actapol.net/volume6/issue4/abstract-7.html.
Serino, A., & Salazar, G. (2018). Protective Role of Polyphenols against Vascular
Inammation, Aging and Cardiovascular Disease. Nutrients, 11(1). https://doi.org/
10.3390/nu11010053.
Squillaro, T., Schettino, C., Sampaolo, S., Galderisi, U., Di Iorio, G., Giordano, A., &
Melone, M. A. B. (2018). Adult-onset brain tumors and neurodegeneration: Are
polyphenols protective? Journal of Cellular Physiology, 233(5), 3955–3967. https://
doi.org/10.1002/jcp.26170.
Tresserra-Rimbau, A., Medina-Rem
´
on, A., P
´
erez-Jim
´
enez, J., Martínez-Gonz
´
alez, M. A.,
Covas, M. I., Corella, D., Salas-Salvad
´
o, J., G
´
omez-Gracia, E., Lapetra, J., Ar
´
os, F.,
Fiol, M., Ros, E., Serra-Majem, L., Pint
´
o, X., Mu
˜
noz, M. A., Saez, G. T., Ruiz-
Guti
´
errez, V., Warnberg, J., Estruch, R., & Lamuela-Ravent
´
os, R. M. (2013). Dietary
intake and major food sources of polyphenols in a Spanish population at high
cardiovascular risk: The PREDIMED study. Nutrition, Metabolism and Cardiovascular
Diseases, 23(10), 953–959. https://doi.org/10.1016/j.numecd.2012.10.008.
Vetrani, C., Vitale, M., Bozzetto, L., Della Pepa, G., Cocozza, S., Costabile, G.,
Mangione, A., Cipriano, P., Annuzzi, G., & Rivellese, A. A. (2018). Association
between different dietary polyphenol subclasses and the improvement in
cardiometabolic risk factors: Evidence from a randomized controlled clinical trial.
Acta Diabetologica. https://doi.org/10.1007/s00592-017-1075-x.
Vetrani, C., Maukonen, J., Bozzetto, L., Della Pepa, G., Vitale, M., Costabile, G.,
Riccardi, G., Rivellese, A. A., Saarela, M., & Annuzzi, G. (2020). Diets naturally rich
in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect
microbiota composition in high-cardiometabolic-risk individuals. Acta Diabetologica,
57(7), 853–860. https://doi.org/10.1007/s00592-020-01494-9.
Willett, W., Rockstr
¨
om, J., Loken, B., Springmann, M., Lang, T., Vermeulen, S.,
Garnett, T., Tilman, D., DeClerck, F., Wood, A., Jonell, M., Clark, M., Gordon, L. J.,
Fanzo, J., Hawkes, C., Zurayk, R., Rivera, J. A., De Vries, W., Sibanda, L. M.,
Afshin, A., Chaudhary, A., Herrero, M., Agustina, R., Branca, F., Lartey, A., Fan, S.,
Crona, B., Fox, E., Bignet, V., Troell, M., Lindahl, T., Singh, S., Cornell, S. E.,
Reddy, K. S., Narain, S., Nishtar, S., & Murray, C. J. L. (2019). Food in the
Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food
systems. The Lancet, 393, 447–492. https://doi.org/10.1016/S0140-6736(18)31788-
4.
Woerdeman, J., van Poelgeest, E., Ket, J. C. F., Eringa, E. C., Sern
´
e, E. H., &
Smulders, Y. M. (2017). Do grape polyphenols improve metabolic syndrome
components? A systematic review. European Journal of Clinical Nutrition, 71(12),
1381–1392. https://doi.org/10.1038/ejcn.2016.227.
L. Condezo-Hoyos et al.
