
Plant Accessible Tissue Clearing Solvent
System (PATCSOS) for 3-D Imaging of
Whole Plants
Hantao Zhang
1
, Lei Zhu
1,2*
, Hu Zhao
3*
, Zhen Li
1,2*
1
College of Biological Sciences, China Agricultural University, Beijing 100193,
China
2
State Key Laboratory of Plant Environment Resilience, College of Biological
Sciences, China Agricultural University, Beijing 100193, China
3
Chinese Institute for Brain Research, Beijing, Beijing 102206, China
*
[email protected] (L.Z.)
Abstract: Tissue clearing is a technique to make the inner structure of opaque tissue
visible to achieve 3-dimensional (3-D) tissue imaging by unifying the refractive
indexes of most of the cell components. Tissue clearing is widely used in animal
tissue imaging, where whole body 3-D imaging has been realized. However, it has not
been widely used in plant research. Most plant tissue clearing protocols have their
disadvantages, including low efficiency, not being fluorescence-friendly and poor
transparency on tissues with a high degree of lignification. In this work, we developed
a new plant tissue clearing method for whole plant imaging, named Plant Accessible
Tissue Clearing Solvent System (PATCSOS), which was based on the Polyethylene
Glycol-associated Solvent System (PEGASOS). The PATCSOS method realized
extensive transparency of plant tissues, including the flower, leaf, stem, root, and seed
of Arabidopsis thaliana, with high efficiency. The PATCSOS method consists of four
main steps: fixation, decolorization/delipidation, dehydration, and clearing.
Subsequently a rapid and efficient clearing of mature plant tissue can be achieved.
With PATCSOS, we can image Arabidopsis seedling in their entirety in 3-D using
endogenous cellulose autofluorescence. What’s more, the PATCSOS method is
compatible with fluorescence protein imaging and GUS staining, which greatly
expands the applicability of this method. We also imaged intact Nicotiana
benthamiana leaf and Zea mays embryos. Our results showed that the PATCSOS
clearing method is an excellent tool to study plant development and cell biology.
Keywords: tissue clearing; Arabidopsis thaliana; PATCSOS; 3-D imaging; confocal
microscopy
1 Introduction
To better investigate plant growth and development, it is important to obtain 3-D
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
images of plant tissues and observe the inner structure of the plants. Different cell
components have different refractive indices (RI), for example, water has a RI of 1.33,
lipids have a RI of above 1.45, proteins have a RI of above 1.44, plant cell walls have
a RI of 1.42, and oxygen produced by green plants has a RI of about 1.00[1-5]. As a
result of these differences, the plant tissues appear to us to be opaque.
To address the issue of refractive index differences, the tissue transparency
technique was adopted a method of making the entire tissue transparent by unifying
the RI of most cellular components. Existing tissue clearing methods can be divided
into two categories: organic solvents and aqueous solution system[5, 6]. For example,
Visikol and Benzyl benzoate/benzyl alcohol (BABB) belong to the organic solvent
system [5, 7, 8]. In the BABB system, organic solvents, including benzyl alcohol and
benzyl benzoate, were used. iTOMEI, ClearSee and its derivative clearing methods,
and Pea-CLARITY used aqueous solutions[9]. These methods have many
disadvantages, such as poor transparency, long processing times, not being
fluorescence-friendly, and, most importantly, not being optimized for clearing plant
tissues.
In 2018, Hu Zhao and his team developed an effective tissue clearing method for
mouse whole body imaging called PEGASOS. PEGASOS can clear a wide range of
mouse tissues and PEGASOS system can protect the fluorescence signal from being
quenched [4]. Based on PEGASOS, we developed a plant accessible tissue clearing
solvent system (PATCSOS) and achieved considerable transparent effect for the whole
plant of Arabidopsis, which can warrant the construction of 3-D images of plant
tissues using autofluorescence from cellulose. We further confirmed that the modified
PEGASOS method is compatible with imaging with fluorescence protein and GUS
staining in plants.
2 Materials and Methods
2.1 Plant materials and growth condition
Arabidopsis thaliana (A. thaliana) accession Columbia-0 (Col-0) and transgenic
line ANTPro::GFP, DR5::GUS [10] and HB29Pro::GUS were constructed (see
below)The Arabidopsis seeds were surface-sterilized for 15 min in 0.5% sodium
hypochlorite and sown on 1/2 Murashige & Skoog medium containing 0.9% plant TC
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
agar (PhytoTechnology Laboratories, Shawnee Mission, KS, USA). The Arabidopsis
grew at 22°C under a 16 h: 8 h, light: dark photoperiod.
2.2 Molecular cloning and transformation
A 2-kb region of the HB29 (AT1G69600) promoter and 2-kb region of the ANT
(AT4G37750) promoter were amplified. HB29 promoter was inserted into
pCAMBIA1391. ANT promoter, GFP gene and NOS terminator were inserted into
pCAMBIA1300 vectors to prepare HB29 Pro::GUS and ANT Pro::GFP constructs.
HB29 Pro::GUS and ANT Pro::GFP constructs were generated and transformed into
Col-0 plants to produce the marker lines. The plant transformation vector
pCAMBIA1300 and pCAMBIA1391 was used to generate transformed plants. Plants
were transformed using Agrobacterium tumefaciens strain GV3101 using the floral
dip method. [11]. The sequences of primers were listed in Table S1.
2.3 Preparation of PATCSOS solutions
The clearing solutions was adopted from PEGASOS with some modifications[4].
Fixation solution. Fixation solution was prepared by mixing 4% (w/v)
Paraformaldehyde (PFA, Sigma-Aldrich 158127) with 0.01 M PBS (pH 7.4, Solarbio
P1003).
Gradient tB solution.30%, 50% and 70% Tert-Butanol (tB, Aladdin T119717) in
water v/v and 3% w/v Quadrol (Sigma-Aldrich 122262) was added afterwards.
tB-PEG dehydration solution. 70% v/v Tert-Butanol (Aladdin T119717), 27% v/v
PEG methacrylate Mn 500 (PEGMMA500, Sigma-Aldrich 447943) and 3% w/v
Quadrol (Sigma-Aldrich 122262).
BB-PEG clearing medium. 75% v/v benzyl benzoate (BB, Aladdin B400547), 25%
v/v PEGMMA500 (Sigma-Aldrich 447943), and 3% w/v Quadrol (Sigma-Aldrich
122262).
2.4 Passive immersion procedure
Seedlings. Arabidopsis seedlings were immersed in 1 mL fixation solution and
fixed overnight at room temperature. The seedlings were washed with 1 mL of ddH
2
O
at 37℃ under gentle shaking for 20 minutes and repeated three times. Then they were
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
immersed in a 30% tB solution for one day at 37℃ under gentle shaking to remove
the chlorophyll. Afterwards, the 30% tB solution was replaced with 50% tB for one
day and then with 70% tB for another day, both at 37℃ under gentle shaking. The
tissues were placed in a dehydration solution overnight at 37℃ under gentle shaking.
Finally, the dehydrated tissues were immersed in clearing medium for at least 24 h at
37℃ under gentle shaking.
Mature tissues. For mature tissues, the procedures were modified to fit the large
diameter and cellulose rich nature of the tissue. For bolting Arabidopsis, the
inflorescence stem was immersed in 4% paraformaldehyde (PFA) overnight at room
temperature, the tissues were washed three times with ddH
2
O, and then immersed in
30% tB for two days, and the 30% tB solution was replaced twice a day to better
remove chlorophyll. The stems were immersed in 50% tB for one day, 70% tB for one
day, and a dehydration solution for one day. And at last, they were immersed in the
clearing medium for at least one day. All above, the steps were performed on a
37℃shaker.
2.5 GUS Staining
Seven-day-old HB29pro::GUS seedlings were used to test compatibility between
PATCSOS and GUS staining. Seedlings were immersed in GUS assay solution (Table
S2) at 37℃ in the dark for 4 hours using a widely adapted protocol [12].
In the decolorizing step, the seedlings were immersed in 30% tB for 10 minutes,
50% tB for 10 minutes and 70% tB for 10 minutes to adapt to the PATCSOS system,
while in traditional methods, ethanol and acetate solution was used.
2.6 Microscopy and image analysis
GUS imaging was acquired on a Leica AF 6000 stereoscopic microscopy.
3-D fluorescence imaging of plant tissues was acquired on a Leica STELLARIS 5
confocal microscopy (Laser lines: 488, 561, 638; Leica HyD photon detector).
Raw images were collected in lossless 8-bit TIFF format. Single GFP
fluorescence images were processed with Fiji (NIH). 3-D reconstructed images were
generated using Imaris 9.0 (Bitplane). Stack images were generated using the “volume
rendering” function. 3-D image clipping was realized using the “Clipping Plane”
function. Background blocking was realized using the “Oblique Slicer” and “Ortho
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
Slicer” function. 3-D pictures were generated using the “Snapshot” function. Movies
were made using the “Animation” function.
3 Results
3.1 Optimization of the PATCSOS procedure and evaluation of
clearing efficiency on plant tissues
There are five to six steps in the PEGASOS protocol, i.e.: fixation,
(decalcification), decolorization, delipidation, dehydration, and clearing [4]. The
decalcification step was omitted due to the absence of bone in the plant tissue. The
decolorization step can also be omitted as there is no heme in the plant tissue and the
gradient tB solution also has the effect of removing chlorophyll, therefore the steps of
delipidation and decolorization can be combined to reduce the complexity of the
method. The optimized PATGSOS protocol for plant tissue clearing included the
following steps: fixation, decolorization/delipidation, dehydration, and clearing.
Mature Arabidopsis inflorescence stems (Fig. 1b, 1e) became completely
transparent after 7 days in the clearing solution, the clearing duration is even shorter
for seedlings (Fig. 1a, 1d). After clearing, the inner vascular bundles can be seen
clearly in the inflorescence stem, and seeds in silique are also visible. This shows that
the pericarp has been completely cleared. Thinner plant tissues, such as leaves and
flowers, got the best transparent effect; they were completely invisible in the clearing
solution after processing (Fig. 1e).
We also cleared Nicotiana tabacum leaf and Zea mays embryo after germination.
The chlorophyll in tobacco leaf was removed effectively, and the whole leaf was
totally transparent after clearing (Fig. 1c, 1f). The embryo and endosperm of corn
seed was removed after clearing to get a better view of the corn embryo (Fig. 1g,1 h).
And after magnifying the embryo with a stereoscopic microscope, we can see the
developing plumule inside the coleoptile (Fig. 1i, 1j).
3.2 3-D imaging with autofluorescence
Autofluorescence is a normal phenomenon in plant tissues. Many cell
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
components such as chlorophyll and cellulose can emit autofluorescence.
Autofluorescence is generally regarded as interference signal in fluoresce imaging and
various methods have been developed to remove it [13, 14]. But it serves as a natural
marker to show the cell profile and plant tissue structure. After clearing, we can
capture autofluorescence signal from cellulose clearly, with which a 3-D image of
plant tissue was obtained (Fig. 2).
We imaged flower of Arabidopsis with autofluorescence using 10×objective in
two channels (excitation: 488 nm and 561 nm), the step size was set at 1 m. Two
single slices at different Z-axis positions showing pollens in the anther sac and ovules
in the ovary, respectively (Fig. 2a, 2b). Clear observation of such tissues indicated
applicability of the PATCSOS for clearing flowers tissues. Reconstructed 3-D images
of flowers (Fig. 2e, 2f) can help us track the direction of the vascular system from
pedicel to stamens and pistil of the flower. So, the PATCSOS system can realize high
resolution 3-D imaging of fine structures of plants, regardless of inner or outer region
without dissection. Whole tissue clearing and 3-D imaging is more intuitive and can
maintain the relative position of each part compared with traditional dissection
methods [15].
More tender and more mature silique tissues were also imaged. Fig. 2c is one of
the slices of the tender silique captured with 40×objective stimulated by 488 nm
laser with a step size of 1m. Because of limited working distance of objective, we
only imaged half of a silique. The slicer tool in Imaris was used to reveal inner
structure of the silique, and the development condition of the embryo can be clearly
observed through the reconstructed image (Fig. 2g). Seeds in different developmental
stage and their relative positions in the silique can be clearly visualized (Fig. 2g1-g4).
A 20× objective with long working distance was used to image another more
mature silique with a small piece of stipe (Fig. 2i). Regretfully, because of the
flavonoid of the testa, the inner structure of some seeds was not clearly visible (Fig.
2i). Images and movie of silique in 3-D view showed continuous change from the
outer silique to the inner, and we can see the arrangement of seeds in a silique (Fig 2k,
Movie S1).
Part of inflorescence stem was imaged with a 10×objective stimulated by 488
nm laser, the step size was set as 1 m (Fig. 2d, 2h, 2j, 2l). The structure of vascular
bundles was clearly countable from the rendered 3-D image (Fig. 2l). The diameter of
the inflorescence stem was about 1 mm, the tissue needs to be highly transparent to be
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint

imaged at such high resolution and quality. This result showed that the PATCSOS
protocol has good clearing effect on Arabidopsis tissues imaging.
3.3 GFP fluorescence imaging
Fluorescence protein (FP) labelling is widely used to investigate tissue
development and protein subcellular localization in cell biology [5]. In whole tissue
clearing analysis, the ultimate goal was to image the inner structure of tissues using
fluorescence; thus, the protection of fluorescent proteins during clearing is of critical
importance.
The passive immerse process employed by PATCSOS can readily protect the
fluorescent proteins by using gradient tB to replace ethanol in traditional plant
material processing methods and adding Quadrol to keep a basic environment to
maintain activities of the FPs. However, GFP imaging of Arabidopsis line ANT
Pro::GFP presented the dilemma of discriminating fluorescence signal between
autofluorescence and GFP. Fluorescence from FPs can only be stimulated by
excitation light with specific wavelength. In plants, chlorophyll and lignin stand out as
the key auto fluorescent substances, yet a variety of additional compounds exhibit
autofluorescence when subjected to ultraviolet or visible light excitation,
encompassing elements from the cytoplasm and the structural cell walls [16]. While
for autofluorescence, a broad wavelength range can be used for excitation. Thus,
during image acquisition, we collected data from two channels, the first channel used
488 nm laser for excitation and signals from both GFPs and autofluorescence were
acquired, the other channel used 561 nm laser for excitation and signal only from
autofluorescence was acquired. Images from these two channels were subtracted to
obtain images only from the fluorescence proteins [17].
Fluorescence images from one section of Arabidopsis ANTPro::GFP stipe was
imaged with excitation laser at 488 and 561 nm, the images showed distinct
distribution of fluorescence signals from GFP and autofluorescence (Fig. 3a , 3b).
When a 488 nm laser was used, fluorescence from both GFP and autofluorescence can
be clearly visualized from cambium and epidermis region of the stipe (Fig. 3a). When
a 561 nm laser was used, fluorescence only from epidermis can be imaged (Fig. 3b).
Since ANT is a marker gene to label the cambium[18], the ANTPro::GFP construct
should emit fluorescence signals only from the cambium region, but fluorescence
image from the 488 nm channel showed ubiquitous distribution of signal due to
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint

interference from autofluorescence from cellulose (Fig. 3a). We further merged
images from the two channels (Fig. 3d), to distinguish autofluorescence from GFP
signal, the orange region represents autofluorescence signal from the 488 nm and 561
nm channels and the green region represents GFP signal from only the 488 nm
channel.
We further used “Subtract” function of the Fiji software remove autofluorescence
interference signal. The brightness of the two images was adjusted to the same level,
and the “Subtract” function was used to performance subtraction between Fig. 3a and
3b, which resulted in a clean GFP fluorescence image of Arabidopsis cambium (Fig.
3e).
Through 3-D imaging reconstruction and background subtraction, a constant ANT
express pattern in the whole stipe was observed (Fig. 3c, 3f), where, the yellow part
represents autofluorescence and the green part represents GFP signal, and the
cambium pattern in the stipe can be clearly visualized.
3.4 Whole seedling 3-D imaging
3-D imaging of whole Arabidopsis seedling was also realized with PATCSOS.
The immature development status of cell wall makes the seedling tissue tenderer than
mature inflorescence stem, turgescences from vacuole provides support for the cell
instead of cellulose in cell walls. Thus, maintaining cell shape during the clearing
process is the critical step for clearing seedlings. We modified the PATCSOS protocol
by shortening the decolorization/delipidation time and controlling dehydration time
strictly to fit the tender nature of seedlings and achieved excellent clearing effect
while maintaining cell shape. Hence, the shoot apical meristem (SAM) and trichome
can stay in good status after clearing (Fig. 4i, 4j). Eight-day Col-0 seedlings were
cleared with the modified PATCSOS method and loaded onto a glass slide with a
central groove. The whole seedling was imaged with a 40×oil objective using 488
nm excitation laser with a step size of 1 m. The image of the seedling vascular
bundle showed that the annular vessel and spiral vessel are the major vessel elements
in the vascular bundle (Fig. 4a). An image of part of the shoot apical meristem (SAM)
clearly showed cell configuration details of the bud primordium (Fig. 4b). When the
laser was focused at the root platform, the root hair could be clearly visualized (Fig.
4c, 4c1), when the laser was focused at the SAM platform, bud primordium could be
clearly visualized (Fig. 4d, 4d1), both images were processed by the brightness adjust
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
function of the Fiji software. 3-D imaging of whole seedling showed clear details of
all the root hairs, which also proved that small details can be well preserved by
processing with PATCSOS (Fig. 4e, 4f, 4g). A movie was made to show more details
of the whole seedling (Movie S2). A slice image in the z-direction of the diarch stele
in Arabidopsis root showed the xylem and phloem arrangement (Fig. 4h). Details of
the surfaces of cotyledon and SAM can be clearly visualized, and the structure of the
trichomes was also well-preserved (Fig. 4j). The modified PATCSOS method realized
whole tissue 3-D imaging of Arabidopsis seedling without the need of dissection,
which is perfectly suitable for 3-D imaging of small and tender tissues such as
seedlings, as such tissues are generally very soft and small in diameter which render
them difficult to dissect/slicing.
3.5 GUS staining after clearing
GUS staining is a widely used technology to reveal gene expression position and
expression level. But in some non-cleared tissues, GUS staining may be difficult to
observe due to the opaque nature of plant tissues. Thus, the combination of GUS
staining and tissue clearing is a perfect way to realize GUS staining from deep-buried,
opaque tissue regions. Here, we tested the compatibility of the PATCSOS system with
GUS staining. Eight-day seedlings were first stained with conventional GUS staining
solution [19]. To adapt to the PATCSOS system, the tissues were decolorized at 30%,
50%, and 70% tB for 10 minutes, respectively, instead of ethanol in the conventional
GUS staining protocol. The decolorized seedling can be cleared in less than 12 h by
immersing in a standard PATCSOS clearing solution.
HB29 is a gene we were concerned about at the earlier time. And DR5 is a famous
auxin respond element.[20] So, we chose these two genes to test our protocol.
HB29Pro::GUS seedling was first GUS stained and then processed with
PATCSOS-GUS clearing, the difference in contrast between tissues is more clearly
(Fig. 5a, 5c). DR5::GUS seedling were also treated with GUS staining and then with
PATCSOS-GUS clearing (Fig. 5b, 5d), some edge areas with unclear coloring in
traditional GUS staining were now clearly visible. Thus, the PATCSOS-GUS clearing
system can maintain GUS staining after clearing and the higher contrast between GUS
staining and cleared tissue makes high resolution 3-D imaging available.
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
4 Conclusions and Discussion
In this work, we modified the popular mouse tissue clearing method PEGASOS
to suit application in plant science research and developed the PATCSOS protocol.
The PATCSOS procedure is FPs friendly and can maintain tissue structure of tender
Arabidopsis seedlings. We also confirmed that PATCSOS is compatible with GUS
staining using the modified PATCSOS-GUS protocol.
With the developed method, 3-D images of different plant organs were acquired.
A new concept for autofluorescence imaging was proposed, which uses
autofluorescence from cell wall components for 3-D imaging, and the
autofluorescence signal has generally been considered as noise signal. With
autofluorescence, 3-D image from an intact seedling was obtained. By acquiring
fluoresce signals from different excitation channels, fluorescence signals from
autofluorescence and FPs can be discriminated to reveal “true” signal from the FPs, in
this way we imaged the cambium pattern in Arabidopsis stipe.
The PATCSOS protocol still has some disadvantages, tB was used for
decolorization/delipidation, but, compared with ethanol, the chlorophyll removing
efficiency of tB is much lower, but ethanol will quench the FPs fluorescence. So, it is
necessary to find a fluorescence friendly and effective chlorophyll removing reagent.
And for seeds, which is regarded as the most difficult organ to be cleared, our current
protocol still has difficulties to fully clear seeds. Light-sheet microscopy is an ideal
technology to image whole mature plant, but we found that the light beam was
scattered badly by the vascular tissue. Thus, a better method to control light scattering
by the vascular bundle is needed to realize 3-D imaging with light-sheet.
5 Supplementary Materials
The following are available in supplementary document.
Table S1: Primers used in this study;
Table S2 GUS assay solution (50 ml);
Table S3 X-Gluc mother liquor (250 μL).
Movie S1: 3-D imaging of Silique;
Movie S2: Whole seedling 3-D imaging presentation.
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint

6 Author Contributions
Zhang HT. designed the project, performed the experiments and imaging, and
wrote the original draft; Li Z., Zhao H., Zhu L. reviewed and edited the article. All
authors have read and agreed to the published version of the manuscript.
7 Acknowledgments
We thank Beijing Students' Platform for innovation and entrepreneurship training
program for providing apart of financial support. We also thank Yutao Wang, Huan
Zhao, Hongjie Xing (China Agricultural University) for help in construction of
transgenic plant material, and Youqi Li, Yuling Wang, Manyu Chen, Jiayi Ding
(Chinese Institute for Brain Research, Beijing) for the imaging technology assistance.
8 Conflicts of Interest
The authors declare no conflict of interest.
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
References:
[1]. Tainaka, K., et al., Whole-body imaging with single-cell resolution by tissue decolorization. Cell,
2014. 159(4): p. 911-24.
[2]. Treweek, J.B. and V. Gradinaru, Extracting structural and functional features of widely
distributed biological circuits with single cell resolution via tissue clearing and delivery vectors. Curr
Opin Biotechnol, 2016. 40: p. 193-207.
[3]. Tuchin, V.V., et al., Light propagation in tissues with controlled optical properties. J Biomed Opt,
1997. 2(4): p. 401-17.
[4]. Jing, D., et al., Tissue clearing of both hard and soft tissue organs with the PEGASOS method.
Cell Res, 2018. 28(8): p. 803-818.
[5]. Heriche, M., et al., Imaging plant tissues: advances and promising clearing practices. Trends Plant
Sci, 2022. 27(6): p. 601-615.
[6]. Silvestri, L., et al., Clearing of fixed tissue: a review from a microscopist's perspective. J Biomed
Opt, 2016. 21(8): p. 081205.
[7]. Villani, T.S., A.R. Koroch and J.E. Simon, An improved clearing and mounting solution to
replace chloral hydrate in microscopic applications. Appl Plant Sci, 2013. 1(5).
[8]. Lee, K., et al., Macro optical projection tomography for large scale 3D imaging of plant structures
and gene activity. J Exp Bot, 2017. 68(3): p. 527-538.
[9]. Sakamoto, Y., et al., Improved clearing method contributes to deep imaging of plant organs.
Commun Biol, 2022. 5(1): p. 12.
[10]. Ulmasov, T., et al., Aux/IAA proteins repress expression of reporter genes containing natural and
highly active synthetic auxin response elements. Plant Cell, 1997. 9(11): p. 1963-71.
[11]. Clough, S.J. and A.F. Bent, Floral dip: a simplified method for Agrobacterium-mediated
transformation of Arabidopsis thaliana. Plant J, 1998. 16(6): p. 735-43.
[12]. Zhao, H., et al., HOMEOBOX PROTEIN 24 mediates the conversion of indole-3-butyric acid to
indole-3-acetic acid to promote root hair elongation. New Phytol, 2021. 232(5): p. 2057-2070.
[13]. Kurihara, D., et al., ClearSee: a rapid optical clearing reagent for whole-plant fluorescence
imaging. Development, 2015. 142(23): p. 4168-79.
[14]. Mizuta, Y., D. Kurihara and T. Higashiyama, Two-photon imaging with longer wavelength
excitation in intact Arabidopsis tissues. Protoplasma, 2015. 252(5): p. 1231-40.
[15]. Jing, D., et al., Tissue Clearing and Its Application to Bone and Dental Tissues. J Dent Res, 2019.
98(6): p. 621-631.
[16]. Donaldson, L., Autofluorescence in Plants. Molecules, 2020. 25(10).
[17]. Yi, Y., et al., Mapping of individual sensory nerve axons from digits to spinal cord with the
transparent embedding solvent system. Cell Res, 2024. 34(2): p. 124-139.
[18]. Schrader, J., et al., A high-resolution transcript profile across the wood-forming meristem of
poplar identifies potential regulators of cambial stem cell identity. Plant Cell, 2004. 16(9): p.
2278-92.
[19]. Zolman, B.K., et al., Identification and characterization of Arabidopsis indole-3-butyric acid
response mutants defective in novel peroxisomal enzymes. Genetics, 2008. 180(1): p. 237-51.
[20]. Ulmasov, T., et al., Aux/IAA proteins repress expression of reporter genes containing natural and
highly active synthetic auxin response elements. Plant Cell, 1997. 9(11): p. 1963-71.
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
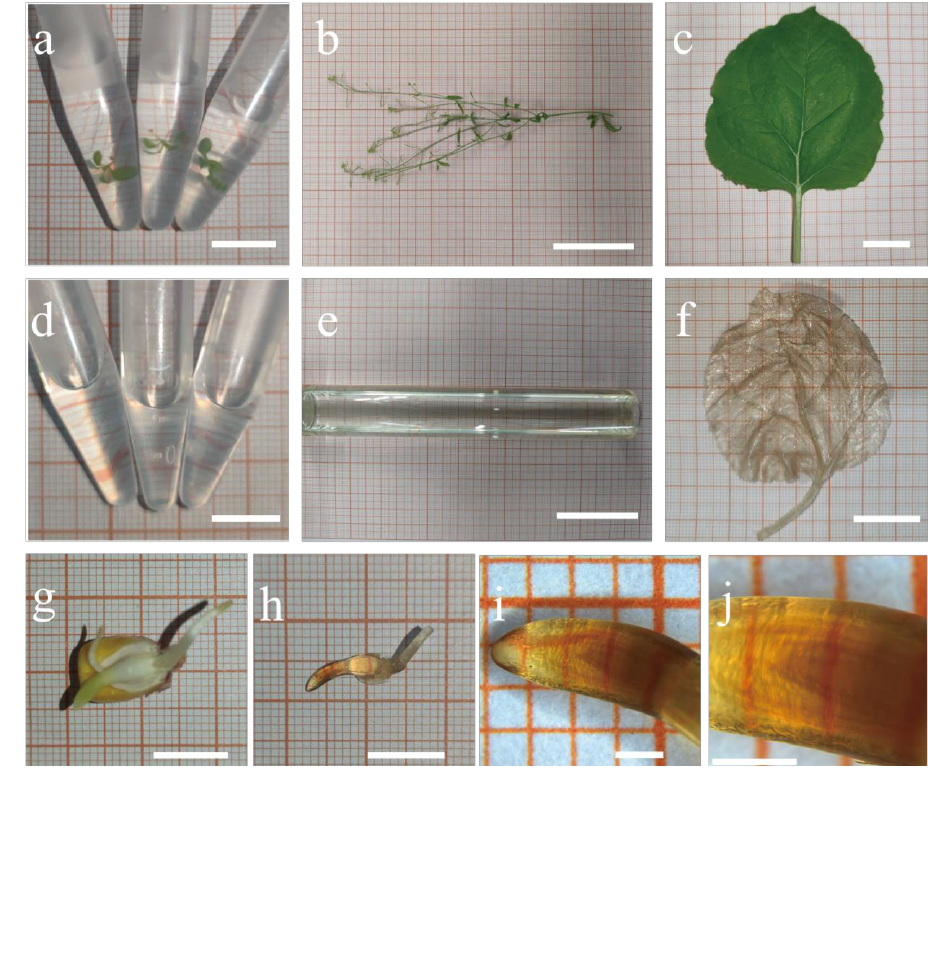
Figure 1. Different plant materials treated by PATCSOS. Arabidopsis seedlings were visible in PFA (a)
without clearing, and after PATCSOS treatment, the seedlings became invisible (d). So as the mature
Arabidopsis plant, a part of the inflorescence stem(b) also can be cleared by PATCSOS (e). Nicotiana
tabacum leaf (c) and germinated Zea mays embryo (g) before (c, g) and after (f, h) processed with
PEGASOSFP. At the center of the embryo, the developing plumule can be seen (i, j). Bar, 1 cm (a, d, g,
h); bar, 5 cm (b, e); bar, 2 cm (c, f); bar, 1 mm (i, j).
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint

Figure 2, 3-D imaging of Arabidopsis tissues using autofluorescence: (a-b) two images of single light slices of
flowers with different focal planes; (e-f) 3-D reconstructed images of flowers; (c) single slice of silique; (g) 3-D
reconstructed image to highlight the embryo; g1-g4: magnification of the image framed by the white dotted line
in (g); (i) single slice of a mature silique; (k) 3-D reconstructed image of the mature silique in (i); (d, h) single
slices of inflorescence stem focused on different depth of focus; (j, l) different view of the inflorescence stem
3-D image, and vascular bundles are labeled by arrows in (l).
Most autofluorescence was excited using 488-nm
argon lasers, and the flower tissue imaging was also excited using 561-nm laser. Bar, 1 mm (a, b, c, d, h, i, k);
bar, 300 μm (e, f, g, l); bar, 100 μm (g1, g2, g3, g4); bar, 500 μm (j).
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint

Figure 3 Confocal imaging of the stipe of ANTPro::GFP transgenic Arabidopsis. The GFP
fluorescence (a) and the autofluorescence (b) imagines are captured. And merge (d), subtract (e) and
view them in 3-D vision (c, f). Two channels were used for imaging. Channel 1 used 488-nm laser as
exciting light, and emission light was collected from 499.8-579.3 nm. Channel 2 used 561-nm laser as
exciting light, and emission light was collected from 623.5-725.7 nm. Bar, 250 μm (a, b, d, e); bar, 200
μm (c, f).
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint

Figure 4 3-D imaging of Arabidopsis whole seedling: (a) vascular bundle in hypocotyl; (b) a prat of
SAM; (c, d) two images of single light slices of seedling focused on different depths; (c1) root hairs, a
magnification of the white dashed box in (c); (d1) bud primordium, a magnification of the white dashed
box in (d); (e-g) different views of whole seedling 3-D image; (h) Z direction view of the seedling root;
(i) an image of cotyledon; (j) an image of trichome. Bar, 1 μm (a, b); Bar, 1 mm (c, d, e, f, i); bar, 100
μm (c1, d1, j); bar, 500 μm (g); bar, 300 μm (h).
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint

Figure 5 Comparison between traditional GUS staining and PATCSOS-GUS staining: (a) HB29Pro::GUS
stained by the traditional method; (b) DR5::GUS stained by the traditional method; (c) HB29Pro::GUS
stained by the PATCSOS-GUS method; (d) DR5::GUS stained by the PACTSOS-GUS method. Bar, 1 mm.
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 22, 2024. ; https://doi.org/10.1101/2024.04.20.590386doi: bioRxiv preprint
