
the tree
THE PISTACHIO TREE; BOTANY AND PHYSIOLOGY
AND FACTORS THAT AFFECT YIELD
Louise Ferguson, Vito Polito and Craig Kallsen
he pistachio is the single most successful
plant introduction to the United States in
the twentieth century.
ORIGIN AND HISTORY
The pistachio is native to western Asia and
Asia Minor, where it is still found growing
wild in numerous hot, dry locations in
Lebanon, Palestine, Syria, Iran, Iraq, India,
Southern Europe and the desert countries of
Asia and Africa. It was introduced to Europe at
the beginning of the Christian era. The first
pistachio introductions to the United States
were by the USDA plant exploration service in
1890. The first California introductions were
planted at the Plant Introduction Station in
Chico, California in the Northern Sacramento
Valley in 1904.
BOTANICAL CLASSIFICATION
The pistachio of commerce is the only edible
species among the 11 species in the genus
Pistacia; all are characterized by their ability to
exude turpentine or mastic. Several are referred
to as pistachios, but the name is generally
reserved for the edible nut of commerce. Its
Latin name is Pistacia vera L. A member of
the family Anacardiaceae, it is related to the
cashew, mango, poison ivy and oak, pepper
tree and sumac.
The tree has a pinnately compound leaf.
Each leaf subtends a single axillary bud. Most
of these lateral axillary buds differentiate into
inflorescence primordia and produce a nut-
bearing rachis the following year; thus,
pistachios bear laterally on one-year-old wood.
Botanically, pistachio nuts are drupes, the same
classification for almonds, peaches, apricots,
cherries and plums. All drupes consist of three
parts; an exocarp, a fleshy mesocarp and an
endocarp that encloses a seed. The difference
lies in the edible portion. In pistachios and
almonds the seed is consumed, rather than the
mesocarp as in stone fruit.
The pistachio tree is dioecious (i.e. two
houses”), meaning the male flowers are borne
on one tree and the female flowers on another.
Therefore, both male and female trees are
required to produce nuts. The female flower is
apetalous (no petals) and has no nectarines,
thus does not attract bees. The pollen is spread
by wind. The pistachio tree is deciduous, so it
loses its leaves in the fall and remains dormant
through the winter.
The rooting habit of the tree is classified as
a phreatophyte. Phreatophytes have extensive
root systems allowing them to mine the soil
deeply. Thus, pistachios are adapted to survive
long periods of drought.
Pistachios are characterized by a long
juvenile period, typically bearing few nuts
before five years of age. They achieve full
bearing between 10 to 12 years of age. The tree
has an upright growth habit characterized by a
strong apical dominance and a lack of lateral
vegetative buds in older trees. These
characteristics have strong implications for
young tree training, mature tree pruning and
rejuvenation of fruiting wood in older trees.
CLIMATIC REQUIREMENTS
Areas suitable for pistachio production have
long, hot, dry summers and moderate winters.
Pistachios grow best in areas with 2200-2800
heat units.
T
31
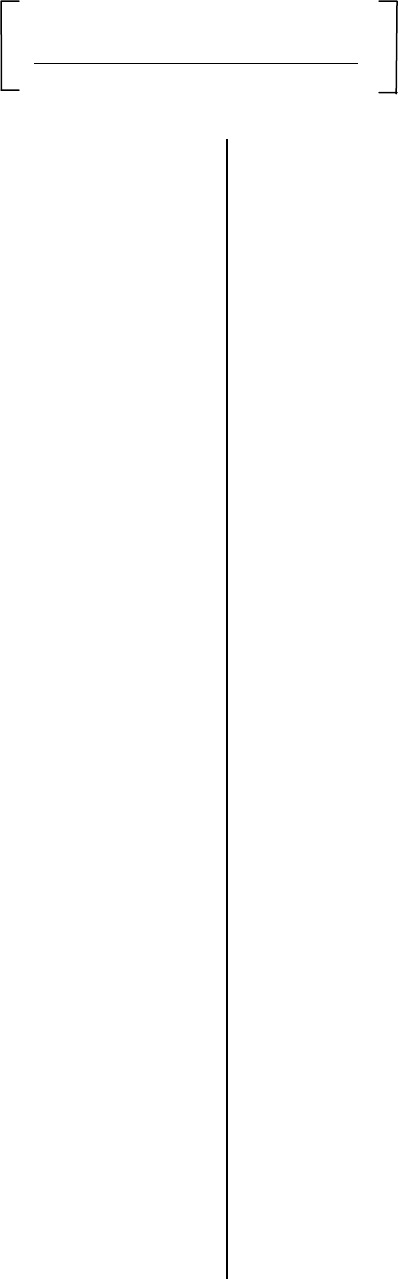
Heat Units = X Number of days
In month (April-Oct)
Mean Monthly Mean Monthly
Tmax
+
Tmin
2
Generally, pistachios should not be planted
above 2500 feet where summer heat is usually
insufficient for complete kernel development.
Elevations of 200 to 800 feet have proven ideal
in the central California valleys. Late spring
rains, frosts, and strong desiccating winds
interfere with pollination. High humidity
through the growing season promotes fungal
diseases that subsequently overwinter on both
male and female trees and reinoculate the tree
the following season. Strong winds are
generally detrimental to young tree training.
Historically, for both the female Kerman
and male Peters to produce good, even, timely
budbreak, normal inflorescences, viable pollen,
good fruit set, and normal vegetative growth,
pistachios in California have required at least
900 winter chilling hours below 45
o
F (7.2
o
C).
When cumulative hours below 45
o
F (7.2
o
C)
have fallen to 670, as they did in 1977-78, the
bloom and foliation have been irregular and
delayed, leaves deformed and yield reduced.
Pistachios can be successfully grown on a
number of soil types. In California, the Pacheco
sandy loams of the southwest San Joaquin
Valley produce the best yields. In areas with
shallow hardpan soils, tree size and
productivity are limited. The tree grows best
on well-drained soils and is intolerant of
saturated conditions. It appears to tolerate
alkalinity and salinity well. The topic of soils
and their modification for pistachio production
is discussed later, in the chapter 4, “Site
Evaluation and Soil Modification” in this
manual.
As stated earlier, pistachios are
phreatophytes and as such can survive harsh
climates without irrigation. Also, the stomata
on their leaves are somewhat less sensitive to
desiccating conditions than stomata on many
other trees. Therefore, pistachios can transpire
a great deal of water under San Joaquin Valley
conditions. The result is a tree that is adapted
for survival, but for economic production,
adequate irrigation is necessary. Irrigation of
pistachios, how much, when, and the method of
application, have important implications for
production. Pistachio irrigation has a
significant impact on young tree development,
soil-borne and aerial diseases, crop yield and
quality (both current and subsequent years) and
tree growth. Irrigation and its impact on these
processes is discussed in chapter 13, “Tree
Water Requirements and Regulated Deficit
Irrigation” in this manual.
SEASONAL PHENOLOGY
The lateral axillary inflorescence buds on one-
year-old wood begin to swell in late March.
Within the first two weeks of April, the 100 to
300 flowers per paniculate rachis are pollinated
and set. Throughout the balance of April and
May the nut shell (endocarp), but not the
nutmeat (seed), enlarges. Through this period
the nut shell is soft and vulnerable to insect
attack and the splitting that appears to be a
result of rain. In June the nut shell hardens, and
from late June through early August the
nutmeat enlarges until it fills the shell. Through
late August and September the nut ripens, the
radial suture around the shell’s long
circumference splits, the hull degrades, and
abscission of the individual nut from the rachis
commences.
Shoot growth is simultaneous with shell
growth. Growth begins in late April and
concludes in late May. The new extension
growth produces pinnately compound leaves
with lateral inflorescence buds in the axils and,
generally, a single apical vegetative bud. The
buds differentiate throughout April, May and
June, become quiescent in July, August and
September, and resume differentiation in
October. Sometimes there is an additional flush
of shoot growth in late June. This growth
produces primarily vegetative lateral buds as
opposed to the inflorescence buds produced by
the spring flush. In August, leaves distal to
heavy fruit clusters often display a marked
depletion and senescence. Most leaves drop by
32

the end of November, and the tree remains
dormant through the following March. As the
trees mature, their strong apical dominance
becomes more marked.
PHYSIOLOGICAL PROBLEMS
Pistachios display three physiological
conditions. The first is alternate bearing; an
annual fluctuation of large crops with poor
crops. The second is the production of blank, or
unfilled nuts. The third is nonsplit nuts, nuts
that fail to split along the lateral nut suture. All
three phenomena appear to be ultimately
related to crop load and are therefore probably
related to carbohydrate competition. Thus far,
little is known about the specific mechanism of
each, though correlation with crop load is
apparent in each case.
Alternate bearing
As stated earlier, pistachios bear laterally on
one-year-old wood. As the trees age, they
develop an alternate bearing pattern with
increasingly large and small crops. Though the
specific mechanism of this phenomenon has
not been defined, evidence suggests that it is a
problem of carbohydrate competition, perhaps
mediated by growth regulator signals. During
the period of nut fill in July, the fruit buds
distal to fruit clusters die and abscise. The
heavier the currently borne crop, the greater the
subtending bud abscission. Thus, following a
heavy crop year, an individual branch may bear
no fruit. Attempts to alleviate the cycle by
nutritional and growth regulator sprays have
not been successful. However, some success in
damping the swing has been achieved with
rejuvenation pruning of older trees. Currently,
pruning appears to be the only method
available to mitigate alternate bearing.
Alternate bearing has not been demonstrated to
harmful to the tree and may therefore only be a
marketing problem. Alternate bearing is not
unique to pistachio trees. Several types of fruit
trees alternate bear. However, only pistachios
appear to possess the phenomenon of
premature bud abscission as the mechanism
that produces alternate bearing.
INTRODUCTION TO BLANK AND
NONSPLIT NUTS
Pistachio fruits consist of a nutmeat (kernel)
enclosed in a thin, hard shell (endocarp)
surrounded by a fleshy hull (mesocarp and
exocarp). The fruit grows from the pistil of the
female flower. The pistil has a single ovary at
its base. The ovary forms the fruit wall that
includes the shell and the hull. Within the
ovary is a single ovule. The ovule, which
contains the female germ cell (egg), will
develop into the edible kernel of the nut.
Extending from the ovary is a three-part style.
Each of the three parts of the style terminates in
a stigma. As the flower opens, these stigma
surfaces become receptive to pollen.
Fruit set typically follows successful
pollination. Pollen is released from staminate
flowers on male trees and is carried by air
currents to stigmas of the pistillate flowers.
When a pollen grain lands on a receptive
stigma surface, it germinates to form a pollen
tube. The pollen tube is an elongate cell that
grows through sigma and style tissue to the
basal ovary and into the ovule. As it does so, it
carries male germ cells to the egg cell. Many
pollen tubes germinate and grow through the
style, but only one successfully reaches the
ovule. That pollen tube enters the ovule and
releases its contents. Fertilization involves the
fusion of a male germ cell with the egg cell.
Thus, the reproductive process leading to fruit
set in pistachio can be seen in three parts:
pollination, involving the transfer of pollen to
the stigma; pollen tube growth, where the male
germ cells are transferred through the stigma
and style to the ovule, and fertilization, the
fusion of the male and female germ cells in the
ovule.
The fusion of the male and female germ
cells produces a single-celled zygote that
eventually grows to form an embryonic plant.
This embryonic plant comprises the kernel.
This process begins slowly, however. The first
division of the zygote does not occur for
several weeks after flowering during which
time the ovary grows to its final size. After
ovary growth is complete, the kernel grows to
fill it. This is an unusual pattern of growth, and
the differential timing of pistachio ovary and
33

kernel development has implications for both
blank nut production and shell splitting.
BLANK NUTS
Blank nuts result when there is fruit set and
ovary growth, but the embryo fails to grow,
leaving the nut shell empty or blank. Blanking
can occur during two different phases of
pistachio nut development, nut setting and nut
filling. It can be affected by crop load and
production practices.
Blanking during nut set
Chronologically, the first empty shells (blanks)
are produced as a result of events that occur at
the time of fruit set. This can occur under a set
of circumstances where pollination occurs but
fertilization fails either because pollen tubes do
not complete growth to the ovule, or the ovule
is not viable when the pollen tubes do arrive.
Under this scenario, the stimulus of pollination
and/or pollen tube growth is sufficient to
induce fruit set, but the failure of successful
fertilization means there is no embryo formed,
so there is no kernel to fill the shell. This
phenomenon of fruit set without fertilization is
called parthenocarpy and is the basis for the
production of several types of seedless fruits,
including some seedless citrus varieties.
Parthenocarpy is fairly common among plants,
however, it normally is found in fruits that have
many seeds rather than in single-seeded fruits
such as the pistachio.
There is some experimental evidence that
pollination-induced parthenocarpy is a
potential mechanism leading to blank pistachio
nuts. In one study, flowers were pollinated with
pollen that had been exposed to a high dose of
gamma radiation. The radiation treatment was
at a threshold level that permitted pollen
germination but inhibited full pollen tube
growth. These experiments resulted in a high
percentage of blank nuts. One explanation is
that the pollination stimulus, which was not
eliminated in the irradiated pollen, is sufficient
to set the fruit, most likely by triggering a
hormonally mediated signal that leads to fruit
set. Research is currently underway to
determine if this finding has implications in the
field.
This form of blanking may be associated
with poor boron nutrition. Boron is known to
be involved in several important aspects of
plant reproductive biology, including pollen
tube growth and ovule longevity, both of which
may have a role in pollination-induced
parthenocarpy. Research has demonstrated
boron leaf levels below 120 ppm dry weight
(August leaf sample) are associated with an
increased percentage of blank nuts at harvest.
Blanking during nut fill
Blanks may also develop in July during kernel
enlargement, when a certain percentage of the
fertilized embryos fail to enlarge to fill the
shell. It is not known what determines the
percentage of nuts filled, but it is suspected the
tree’s stored carbohydrate capacity initially
determines the percentage of filled nuts. This
theory is corroborated by demonstrations that
thinning a cluster prior to nut growth will result
in a higher percentage of filled nuts on the
thinned cluster. However, the thinned and
unthinned clusters have virtually the same
absolute number of filled nuts, though the
thinned clusters may have slightly larger nuts.
This also has been demonstrated on a whole
tree scale with pruning experiments.
Blanking is more sensitive to insufficient
irrigation than is splitting.
Effect of alternate bearing on blanking
Production of blank nuts is strongly affected by
alternate bearing. As can be seen in Table 1,
the percentage of blank nuts is always higher in
the ‘off’ crop year. This is a further, though not
proven, corroboration of the idea that the
carbohydrate status of the tree entering a crop
year sets the limits on the crop load a tree is
able to set and carry through maturation to
splitting. Markedly different crop loads within
a given crop year (as shown in Table 1) had
virtually the same percentage of blank nuts. For
example, in 1989 the control trees with 0.2
pounds of crop per tree had 19.1% blanks,
while the topped and hedged trees with 11.4
pounds of crop had 16.0% blanks. There was
no statistically significant difference in these
percentages despite the difference in crop
loads. Plate 3A shows “on” and “off” current
year pistachio shoots in mid July after axillary
34
Pistachio nuts may split along the
longitudinal ridges of the shell and at the tip of
the shell. Splitting can occur in any
combination of one or both of the longitudinal
ridges, with or without the tip splitting, or at
the tip alone. Investigation of the anatomical
structure of the longitudinal and tip split
regions indicates that these parts of the shell
differ from one another structurally suggesting
that different mechanisms may be involved in
shell separation at each site.
flower bud abscission. The “on” year shoot is
the one with nuts.
Conclusion
At present, there appears to be very little that
can be done to affect the percentage of
blanking. However, maintaining boron leaf
levels above 120 ppm and providing sufficient
water to avoid water stress during the season
will at least avoid exacerbation of blank nut
production.
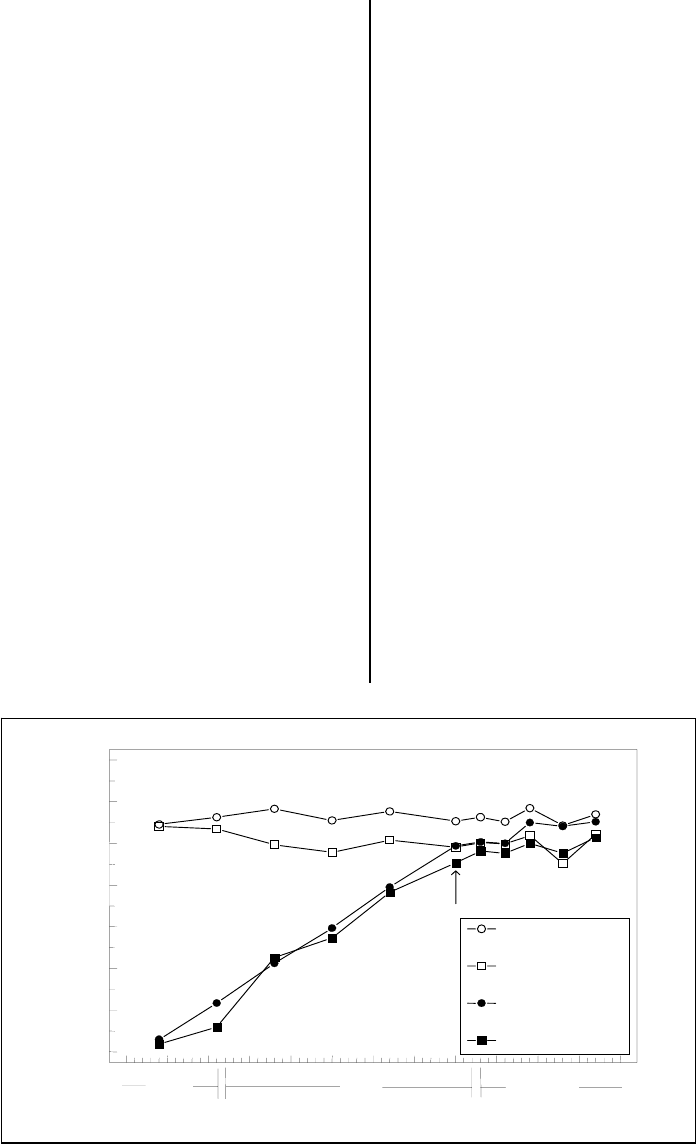
Shell splitting is dependent upon nutmeat
growth and development within the shell.
Figure 3a shows the relationship between shell
split and kernel growth. Note that kernel
growth begins after the shell has reached its
full size. The first split shells are seen about the
time the kernel has grown to fill the shell and
would be exerting physical pressure on it. At
this time, the shell is fully lignified (hardened),
and the cells that form the regions where
longitudinal splitting will occur are dead. This
fact would seem to rule out the possibility that
biochemical factors are involved as a
controlling mechanism in shell split making it
unlikely that a chemical agent to enhance
splitting will be discovered.
NON SPLIT NUTS
The edible pistachio, unlike the species used
for rootstocks, is characterized by splitting of
the nut shell at maturity. Splitting begins about
the end of July, at least one month before fruit
maturity, and continues through mid-
September, progressing simultaneously with
nutmeat maturation. Final nutmeat maturity is
indicated by separation of the hull from the
shell. This is accompanied by a breakdown of
chlorophyll pigments in the hull, allowing the
red pigments to become visible. Thus, the most
obvious indicator of shell splitting is the
appearance of red color in the hull.
Figure 3a. This graph illustrates kernel and shell growth. The major (in the plane of the longitudinal
split lines) and minor (perpendicular to the plane of the longitudinal split lines) diameters are shown.
Data points represent means of 20 samples. The arrow indicates the time split shells were first seen in
any nut (from Polito, V. S. and K. Pinney. 1999. Endocarp dehiscence in pistachio [Pistacia vera L.]
Internat. J. Plant Sci. 160:827-835).
170 175 180 185 190 195 200 205 210 215 220 225 230
Julian Date
0
2
4
6
8
10
12
14
Size (mm)
First
Split
Major Diameter (S)
Minor Diameter (S)
Major Diameter (K)
Minor Diameter (K)
July
June
A
ugust
35
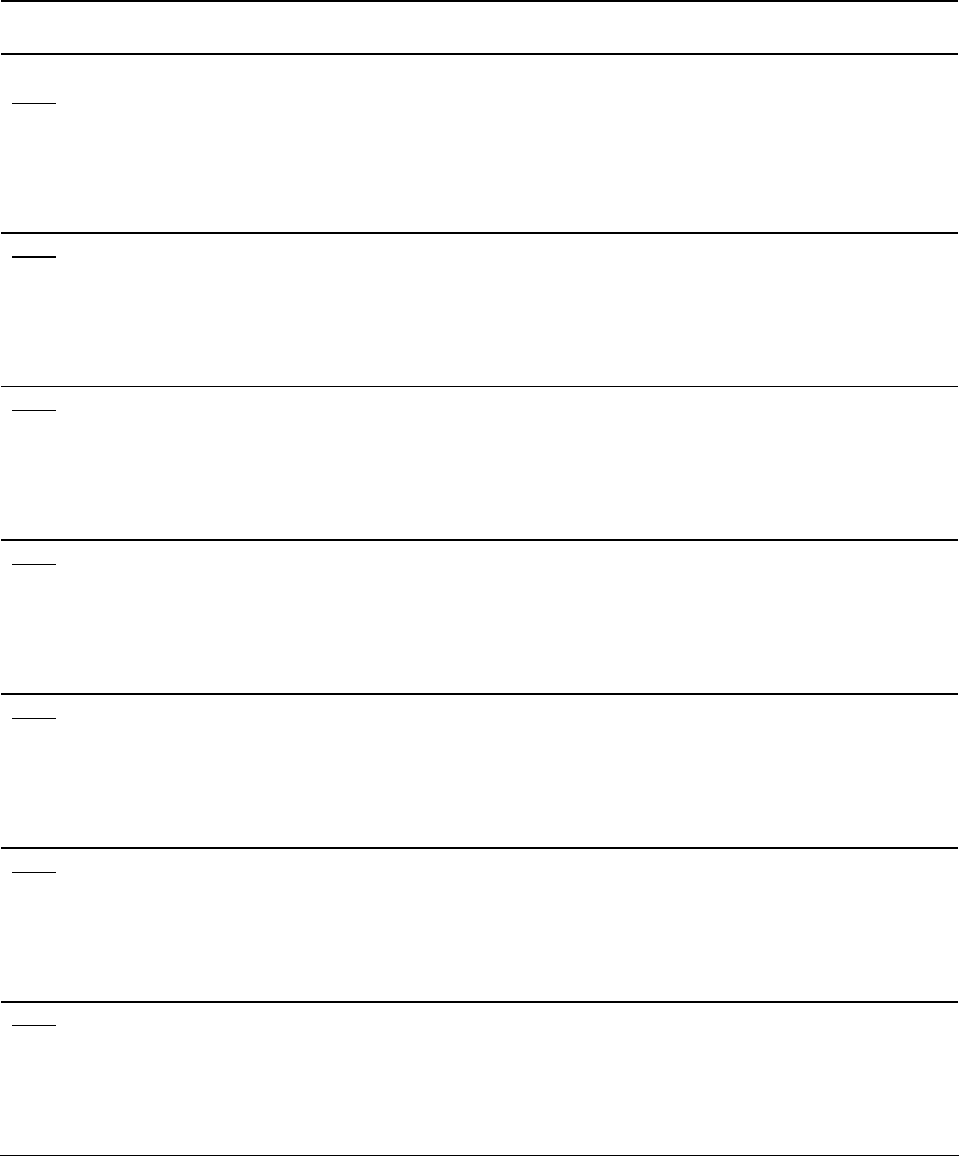
Table 1. Effect of 'on' and 'off' year versus individual tree crop load on the percentages of blank,
split and non-split nuts (by weight).
Year & Non-
Treatment Tree yield Blank Split Split
lbs/tree % % %
1985 'Off'
Control 6.4 a 8.1 a 86.4 a 3.2 a
Hedge 4.6 b 7.2 b 86.1 a 3.0 a
Top 3.7 b 8.4 a 84.9 a 2.9 a
Hedge & Top 1.8 c 8.1 a 85.8 a 2.7 a
Average 8.0 85.8 3.0
1986
'On'
Control 49.2 a 2.1 a 63.4 a 30.4 a
Hedge 44.6 a 2.5 a 65.5 a 28.1 a
Top 39.9 b 2.9 a 64.2 a 30.1 a
Hedge & Top 28.1 c 2.6 a 62.5 a 29.4 a
Average 2.5 63.9 29.5
1987
'Off'
Control 3.5 c 10.1 ab 83.3 ab 3.7 a
Hedge 7.6 b 8.5 b 83.8 a 3.1 a
Top 11.7 a 11.5 a 80.3 b 3.7 a
Hedge & Top 14.2 a 10.2 ab 83.1 ab 3.6 a
Average 10.1 82.6 3.5
1988
'On'
Control 34.1 a 2.7 a 72.9 a 20.3 a
Hedge 26.3 b 2.8 a 72.6 a 21.3 a
Top 24.5 b 2.2 a 72.3 a 22.4 a
Hedge & Top 26.2 b 2.2 a 72.6 a 22.7 a
Average 2.5 72.6 21.6
1989
'Off'
Control 0.2 c 19.1 a 47.6 c 19.1 a
Hedge 0.6 c 17.7 b 61.2 b 13.5 c
Top 6.0 b 14.1 c 63.6 a 15.1 b
Hedge & Top 11.4 a 16.0 a 64.4 a 12.8 c
Average 16.7 59.2 15.1
1990
'On'
Control 37.1 a 11.2 a 67.5 a 21.1 a
Hedge 35.3 a 9.3 a 68.5 a 22.1 a
Top 30.4 b 11.7 a 63.5 a 22.8 a
Hedge & Top 27.2 b 13.8 a 67.4 a 18.7 a
Average 11.5 66.7 21.2
1991
'Off'
Control 3.0 c 19.2 a 59.0 a 21.1 b
Hedge 4.2 c 16.0 a 57.1 ab 26.0 b
Top 17.2 b 15.1 a 50.1 bc 34.0 a
Hedge & Top 25.8 a 17.0 a 47.1 c 35.1 a
Average 16.8 53.3 29.1
* Values within a crop year column followed by the same letter are statistically equal.
36
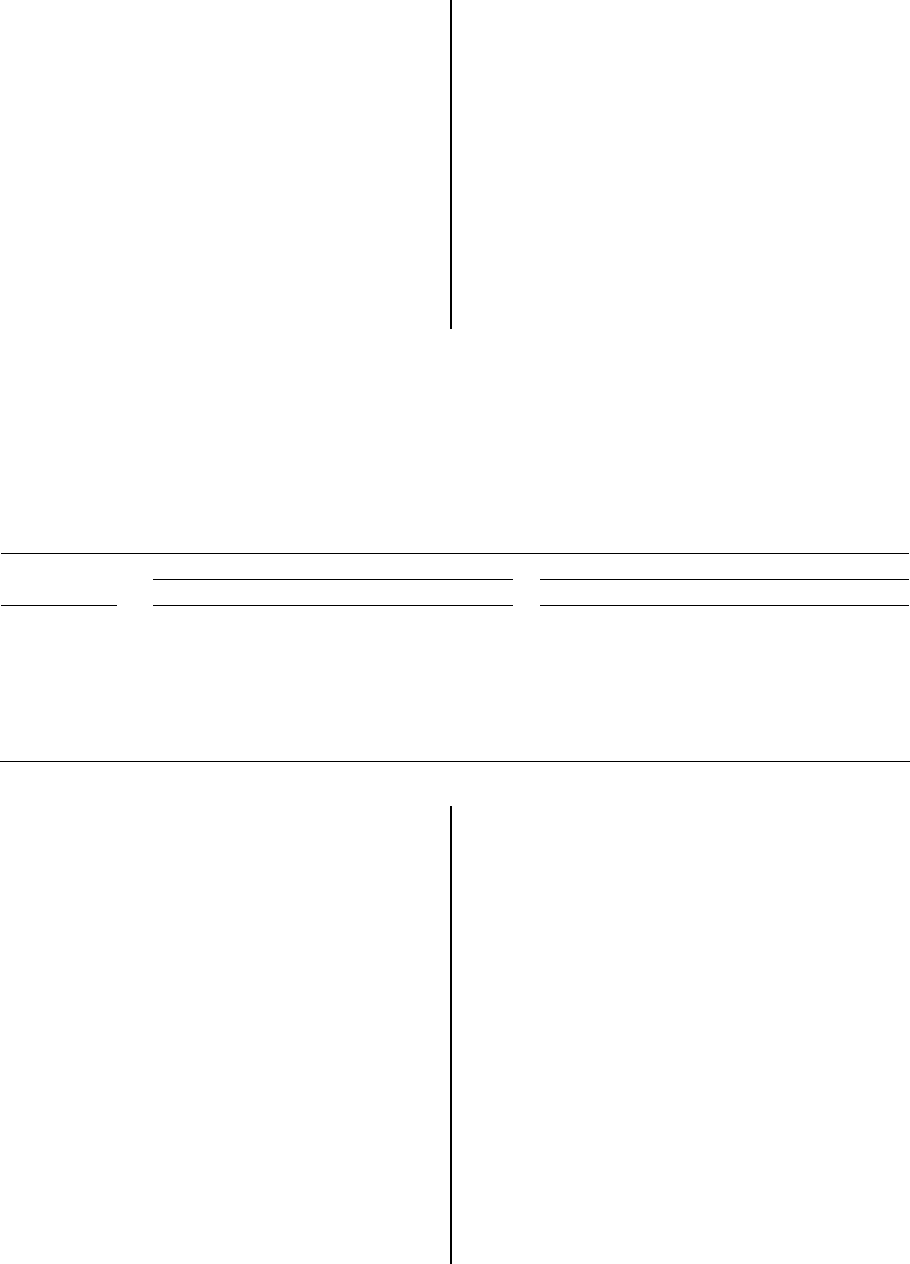
There is a correlation between kernel-to-
shell size ratios and longitudinal splitting.
Table 2 shows the relationship of kernel
diameters to inner shell diameters for six
samples of undried, filled nuts. In each case,
the ratio of kernel to shell size is greater for
fully split nuts than it is for tip-split or unsplit
nuts. Interestingly, there is no statistical
difference between tip-split and unsplit nuts, a
finding that is consistent with anatomical
indications that tip split and longitudinal split
involve different mechanisms. Furthermore, for
fully split nuts, the kernel-to-shell diameter
ratios in the minor axis, i.e. perpendicular to
the plane of the longitudinal ridges, is
consistently greater than one. This means that
for fully split nuts, kernel size is greater than
shell size in the direction where force against
the inside of the shell would tend to drive the
shell halves apart. These correlations would
seem to implicate mechanical force generated
by the growing kernel against the shell as the
mechanism for shell splitting. It should be
noted, however, that experimental evidence to
verify this inference is lacking.
Table 2. Ratio of kernel to shell (K:S) diameters for undried, filled nuts. Nuts were considered in
three categories: nuts that had split fully along both longitudinal ridges, nuts that had split at the tip of
the shell only, and nuts with unsplit shells. Ratios are for kernel diameter and shell inner diameter at
their widest point. The major diameter is in the plane parallel to the longitudinal ridges; the minor
diameter is in the plane perpendicular to the longitudinal ridges. For each sample n = 20; samples 4
through 6 had fewer than 20 tip-split nuts. For each diameter ratio for each sample, values with
different letters differ significantly (P <0.05).
Ma
j
or Diameter (K:S Ratio) Minor Diameter (K:S Ratio)
Sam
p
le Full S
p
lit Ti
p
S
p
lit Uns
p
lit Full S
p
lit Ti
p
S
p
lit Uns
p
lit
1 0.97 a 0.90 b 0.89 b 1.06 a 0.90 b 0.96 b
2 0.98 a 0.85 b 0.85 b 1.07 a 0.90 b 0.90 b
3 0.95 a 0.88 b 0.83 b 1.06 a 0.94 b 0.92 b
4 1.02 a -- 0.90 b 1.10 a -- 0.97 b
5 0.94 a -- 0.83 b 1.02 a -- 0.88 b
6 0.97 a -- 0.81 b 1.02 a -- 0.90 b
If it is the growth of the kernel that drives
shell split, then factors that enhance kernel size
relative to shell size would lead to more split
nuts. Crop load is one such factor; irrigation
management is another. Both can affect the
percentage of split nuts.
There is an inverse relationship between
tree crop load, the percentage of nuts with split
shells, and the percentage of blank nuts (Table
1). As crop load increases, the percentage of
nuts with split shells decreases, and the
percentage of blank nuts decreases. Thus, in
'heavy' crop years the marketable crop is
decreased by non-splits, and in 'light' crop
years it is decreased by blanks. Further, it is
interesting to note that the percentage of non-
split nuts and blanks is much more strongly
correlated with the 'heavy' and 'light' crop year
than with individual tree crop load. A review of
the seven years of crop production in trees with
altered crop loads in Table 1, shows that only
when the crop load is as little as 0.2 pounds per
tree versus 11.4 pounds per tree (1989), or 3.0
versus 25.8 pounds per tree (1991), are
significant differences produced in the
percentage of split shells. This suggests that the
ability of the tree to support and mature a crop
is a stronger factor in determining the
percentage of splitting than the actual pounds
of crop on the tree in any given season.
Effects of female cultivar, pollen
source and rootstock on shell splitting
Research within the past 20 years has
demonstrated the effects of scion cultivar and
pollen source, but not thus far of rootstock, on
37

pistachio nut shell splitting. First, it is well
established that variability in shell splitting
exists among edible pistachio cultivars. This is
one of the primary selection criteria for new
cultivars and was among those for the selection
of 'Kerman'. Second, research demonstrated
pollen from 'Peters' and 'Ask' male trees
produced a higher percentage of split nut shells
than pollen from 'Atlantica' males. There is no
evidence that different rootstocks producing
differences in shell splitting. In rootstock trials
currently being conducted in California, no
significant differences in shell splitting
percentages have been detected among P.
atlantica, P. integerrima and the hybrids of
these two rootstocks.
Effects of preharvest production
practices on shell splitting
Certain field production practices do affect
shell splitting. In decreasing order of degree of
impact, these factors are: harvest time,
irrigation management, boron nutrition and
dormant pruning.
There are research results that show the
highest shell split percentages are achieved
when harvest is delayed until the maximum
number of nuts display hull dehiscence, or
separation, from the nut shell. Dehiscence is
signaled primarily by a hull color change to
red. In practice, progress of hull dehiscence is
evaluated by observing the early color change
in the hulls and, at that time, randomly
sampling trees for split nuts. Specifically,
collect a 100-nut sample from around the tree,
remembering those in the upper southwest
quadrant will mature first, and determine the
percentage of nuts on which the hull is easily
removed and the shell is split. Do this daily
until the increase in the percentage of split nuts
appears to be slowing. However, this period of
maximum nut shell splitting must also be
balanced against the threat of navel
orangeworm (NOW) infestation, (the
possibility of a spray with a preharvest
interval), as well as the availability of
harvesting machinery. Delaying harvest until
maximum nut shell splitting percentages are
achieved may result in NOW infestation and
shell staining.
Additional research has demonstrated
insufficient irrigation from mid-August through
early September will significantly decrease the
percentage of split nuts. Further, preliminary
data currently being generated suggests that
regulated deficit irrigation from mid-May
through the end of June may increase the
percentage of shell splitting.
Studies showed that a late dormant spray of
2-5 pounds of Solubor per acre, applied at
budswell, will significantly increase the
percentage of split nut shells. This can be tank
mixed with the late dormant zinc spray.
Dormant pruning has a negligible effect on
the percentage of splits. The alterations in yield
presented in Table 1 were produced by dormant
pruning. From this data, it can be seen that only
when dormant pruning produced differences in
crop load per tree that varied significantly were
there significant differences in the percentage
of splits, as in 1989 and 1991. Further, these
differences were produced by pruning
treatments done four and seven years earlier.
Later research demonstrated approximately
half a pistachio tree's fruit buds can be
removed, and the tree will compensate by
setting more nuts per cluster with the same
percentage of split nuts as the unpruned
controls. Thus, dormant pruning has a limited
effect on shell splitting; unless the pruning is
quite severe, it does not impact tree crop load,
and therefore will not impact shell splitting.
Further, as discussed previously, shell splitting
appears to be more responsive to 'on' and 'off '
crop year than individual tree crop load.
Thus far, no information has been
generated in California demonstrating the
effects of irrigation water quality on shell
splitting. Thus, it is not ranked in the
production practices discussed here. However,
preliminary information is available from Israel
that irrigation water salinities of 4,000 mg/liter
of total soluble solids (TSS), primarily sodium
(Na) and chlorine (Cl), have decreased shell
splitting. Currently, rootstock trials are in
progress in California to determine the relative
salinity tolerance of P. atlantica and P.
integerrima and the two hybrids of these two
rootstock species, to salinities ranging from
ECws of 0.75 through 8.0.
38

Effects of postharvest factors on shell
splitting
No postharvest practice has yet been
demonstrated to significantly impact the
percentage of split pistachio nut shells.
However, pistachio shells have a high
percentage of moisture. Anytime the harvested
pistachio nut is subjected to heat, during
postharvest transport, preprocessing waits and
drying, this heat will decrease the moisture
content of the shells, literally shrinking the
shell about the nut and increasing the width of
the split. Thus, pistachios can leave the field
with a lower percentage of wide splits than
they have when they arrive at the processor.
During processing, this increase in split width
occurs very early in drying and increases as
dryer temperatures increase from 125
o
F to 190
o
F. This increase can result in nut kernels
dropping out of the shell.
Conclusion
Controllable factors that affect shell split
include: harvest timing, irrigation and boron
nutrition. To obtain the best percentage of split
shells with the 'Kerman' female and 'Peters'
male cultivars: harvest timing should be based
upon the appearance of hull color and a
sampling for the percentage of splits. Trees
should not be water stressed from mid-August
through September; and boron levels should be
maintained above 120 ppm by dry weight of
July leaf sample. Hopefully, through the long-
term rootstock evaluation, and cultivar and
rootstock breeding program in progress,
information concerning the effects of rootstock
and salinity on shell splitting, as well as new
female cultivars and male pollinizers, will be
available in the next few years.
39
