
Back to Table of Contents 5
W E L C O M E
Welcome to the CVICU at Vanderbilt University Medical Center. We are excited to have you join our
team! Our CVICU is comprised of 27 beds that support our medical cardiology and cardiothoracic surgery
teams. Our nurses care for the sickest paents in the region and manage mulple high acuity therapies in-
cluding: various mechanical cardiac assist devices, connuous renal replacement therapy (CRRT), and ECMO.
To accommodate this acuity, we maintain a nurse to paent rao of 1:1 or 2:1. We are proud of the care that
we provide our paents and look forward to equipping you with the skills necessary to provide the excellent
care for which CVICU has become known.
Aer compleng hospital orientaon, you will join us for a 9-12 week orientaon to CVICU, depend-
ing on your clinical background. Our expectaon during this me is that you advocate for yourself and for
your paents by asking thoughul quesons and ulizing the resources provided to you. During your rst day
on the unit, you will meet with the unit educator to review your orientaon plan and materials. In addion to
your precepted me on the unit, you will complete several classes and online learning modules, including
three device-specic classes and a CVICU Boot Camp that will challenge you to apply the knowledge you have
learned. Throughout your orientaon you will meet with your Clinical Sta Leader (CSL) and Educator to
track your orientaon progress and answer any quesons you may have.
Aer you successfully complete orientaon, you will connue to directly report to your CSL. Our lead-
ership team is commied to providing you with professional development and growth opportunies not only
during orientaon but also throughout your career at Vanderbilt. As you develop prociency and condence
in your nursing pracce in the CVICU, we look forward to helping you grow in your own leadership capabili-
es and work toward your specic career goals.
Sincerely,
The CVICU Leadership Team
Kim Carter MSN, RN, CEN - Unit Manager
Kaela Craven, MSN, RN - Interim Nursing Educaon Specialist
Jessica Williams BSN, RN, CCRN - Program Coordinator
Deann Prue, BSN, RN - CSL
Heidi Jo Redenius, BSN, RN - CSL
Michaela Banta, BSN, RN - CSL
Reed Glover, BSN, RN - CSL
Rachel Moore, BSN, RN - CSL
Adam Aycock, BSN, RN - CSL

Back to Table of Contents 6
C O N T E N T S
I. The Vanderbilt Culture: CVICU Guidelines and Expectaons
What is Magnet?.................................................................................................................................... 8
Shared Governance………………………………………………………………………………………………………………………….. 8
Aendance Policy …………………………………………………………………………………………………………………………….. 9
Scheduling Guidelines ………………………………………………………………………………………………………………………. 9
Standards of Care …………………………………………………………………………………………………………………………….. 11
Bedside Report …………………………………………………………………………………………………………………………….….. 12
Code Roles and Responsibilies …………………………………………………………………………………………………….…..12
Escalang Issues ………………………………………………………………………………………………………………………………. 12
Recommendaon Leers………………………………………………………………………………………………………………..... 13
II. Crical Care Foundaons: Care of the Crically Ill Cardiac Paent
Cardiac Structural Anatomy……………………………………………………………………………………………………..………...14
Cardiac Physiology and the Cardiac Cycle………………………………………………………………………………………..….17
Determinants of Adequate Cardiac Output……………………………………………………………………….………………..18
Hemodynamic Monitoring ………………………………………………………………………………………………………………...18
Mechanical Venlaon………………………………………………………………………………………………….……………………22
Arterial Blood Gas Monitoring……………………………………………………………………………………………….…………...25
III. Crical Care Pharmacology
Vasopressors & Inotropes……………………………………………………………………………………………………………………28
Vasodilators and Anhypertensives…………………………………………………………………………..…………………..……29
Inodilators………………………………………………………………………………………………………….……………………………....31
Anarrhythmics and Heart Rate Control……………………………………………………………………………………………..31
Sedaon……………………………………………………………………………………………………………………………………………...34
Neuromuscular Blockade…………………………………………………………………………………………………………………….35
IV. Cardiac Surgery: Nursing Care of the Paent Undergoing Cardiac Surgery
Preoperave Nursing Care …………………………………………………………………………….…………………………………...37
Coronary Artery Bypass…………………………………………………………….………………………………………………………...38
Valve Replacement …………………………………………………………………………………………………………………………….39
Heart Transplantaon ……………………………………………………………………………………………………….……………….41

Back to Table of Contents 7
Lung Transplantaon ………………………………………………………………………………………………………………………. 44
Vascular Surgery………………………………………………………………………………………………………….…………………….45
V. Medical Cardiology: Nursing Care of the Medical Cardiology Paent
Acute Coronary Syndrome (ACS) and Care of the Post-Intervenon Paent…………………………………..… 47
Targeted Temperature Management…………………………………………………………………………………………...…..49
Management of the Heart Failure Paent……………………………………………………………………...………………...51
Cardiomyopathy ……………………………………………………………………………………..………………………………..52
Cardiogenic Shock……………………………………………………………………………………..……………………………………...53
VI. Advanced Therapies and Devices
Temporary Pacemakers ……………………………………………………………………………………………………………...…….55
Intra Aorc Balloon Pump (IABP)……………………………………………………………………………..………………………..57
Impella…………………………………………………………………………………………………………………………...…………………59
Centrimag ……………………………………………………………………………………………………………………..………………… 60
Extracorporeal Membrane Oxygenaon (ECMO)……………………………………………………………………….………61
Ventricular Assist Device (VAD)………………………………………………………………………………………………………….63
Total Arcial Heart ………………………………………………………………………………………………...……………………… 65
VII. Appendix
Case Study Answers & Raonales …………………………………………………………………………………………………… .69
References…………………………………………………………………………………………………………………………………………71

Back to Table of Contents 8
C U LTUR E
The
A N D E R B I L T
V
CV I CU G ui del i n e s a nd Ex p e ctati o ns
I
In this chapter, policies and employee expectaons
are reviewed. For a complete list of hospital poli-
cies, please refer to Policy Tech on the Vanderbilt
Nursing homepage. To nd more informaon on
unit stang and holiday policies, please refer to the
CVICU website.
WHAT IS MAGNET?__________________________
Magnet is a highly coveted designaon granted by
the American Nurses Credenaling Center (ANCC)
to hospitals that promote nursing excellence and
quality paent care (American Nurses Credenaling
Center, 2011). The ANCC (2011) recognizes 4 key
strategies that promote exceponal outcomes:
transformaonal leadership, structural empower-
ment, exemplary professional pracce, and innova-
on. As part of our commitment to exceponal out-
comes, the CVICU leadership team acvely pro-
motes professional development, shared govern-
ance, and diversity.
SHARED GOVERNANCE_______________________
The leadership team expects all nurses to parci-
pate in unit or hospital-based commiees as part
of their commitment to the CVICU. These com-
miees represent an essenal part of the shared
governance structure at Vanderbilt and provide an
opportunity for sta to give input on administrave
decisions. While the scope of each commiee may
vary, all commiees serve to support the connu-
ous improvement of paent care delivery. A list of
nursing-sensive hospital commiees can be found
in the educator’s oce. Descripons of CVICU com-
miees and other opportunies to be involved can
be found in the following secons.
Unit Board
The CVICU Unit Board provides a structure for col-
laborave decision making between CVICU sta and
leadership. Agenda items may come from the sta,
management team, physicians or other disciplines
that serve the CVICU paent populaon. Unit Board
is open to all sta and unit board decisions are
made by consensus agreement. The CVICU Unit
Board meets the rst Tuesday of every month in the
CVICU conference room. A complete copy of the
Unit Board Charter can be found on the CVICU web-
site.
Educaon Council
The CVICU Educaon Council provides a forum to
idenfy educaonal needs of CVICU sta and share
educaonal informaon. Educaon Council Co-
Chairs assist with connuing educaon and act as a
resource for CVICU sta and leadership. Educaon
Council is open to all CVICU sta and management,
and meets on the rst Tuesday of every month. A
complete copy of the Educaon Council Charter can
be found on the CVICU website.
PIPS Champions
On rst and third Tuesday of every month, CVICU
PIPS champions assist members of the leadership
team am and Wound Ostomy Care Nurse (WOCN)

Back to Table of Contents 9
team to idenfy paents at risk for pressure injury
during the Pressure Injury Prevenon Survey (PIPS).
These nurses also serve as resources to sta and
advocates for paents regarding pressure injury in
the crically ill paent.
ATTENDANCE POLICY_________________________
All dayshi sta are expected to clock in between
0638 and 0645. Nightshi sta are expected to clock
in between 1838 and 1845. The CVICU Kronos me
clock must be used to clock in and out. If, for any
reason, an employee is unable to work a scheduled
shi, the employee must speak with the unit charge
nurse before ve o'clock on the designated shi.
Employee me can be checked and approved by vis-
ing the Kronos website, accessible through Quick
Links on the Vanderbilt Nursing website.
Absence and Tardiness
An employee is considered absent when they are
unavailable for their scheduled shi without prior
approved me o. An employee is considered tardy
if they clock in later than the approved mes, leave
work prior to the end of the assigned shi without
prior approval, or fail to clock in at the designated
me clock.
Occurrences
An occurrence is documented as an absence, tardy
or missed me clock in/out. Leadership uses the grid
in Box 1.1 as a guideline when addressing occur-
rences. Occurrences are tracked on a rolling 12-
month period, provided that the reason for an oc-
currence is not covered by FMLA.
SCHEDULING GUIDELINES_____________________
All full-me sta are required to work four weekend
shis per six-week schedule. Full-me sta are also
required to work one weekend call shi and one
weekday call shi per schedule. Sta may not work
more than ve consecuve shis in direct paent
care without manager approval.
Requesng Time O
On dayshi, up to six sta members may be on va-
caon per week. On nightshi, up to ve sta mem-
bers can be on vacaon per week. Sta may request
up to 14 days (six shis) o at a me. PTO is granted
by rst request except for during the summer
months. During the summer, PTO is granted by sen-
iority.
Sta may request preferred o (P-OFF) days when
they would like to be o on a certain day without
taking PTO. Preferred o requests will be consid-
ered unl 1600 on the Wednesday before the
schedule process opens. The scheduling meline is
posted on the CSL oce door. Sta may request up
to six P-OFF days per scheduling period.
OCCURRENCE/
DAYS
DISCIPLINE AND
ACTION
Occurrence
1 Occurrence is:
1 Absence
2 Tardies
2 Missed
Clocks
4 Occurrences
6 Occurrences
8 Occurrences
10 Occurrences
Verbal Warning
Wrien Warning
Final Warning
Terminaon
Days Absent
Consecuve
Non–
Consecuve
6 Days
9 Days
12 Days
15 Days
Verbal Warning
Wrien Warning
Final Warning
Terminaon
No Call/ No Show 1 Occurrence
2 Occurrences
3 Occurrences
Wrien Warning
Final Warning
Terminaon
BOX 1.1

Back to Table of Contents 10
Scheduling Groups
There will be three scheduling groups (Group A,
Group B and Group C). The groups will rotate who
schedules rst during the self scheduling window.
The rst group to sign up during the scheduling pro-
cess will be the last group to be moved from their
requests, the second group will be second to be
moved and the third group the rst to be moved.
Holiday Scheduling
All full-me sta are required to work two major
and two minor holidays. Holiday schedules are de-
termined by sta seniority. Major holidays
(Thanksgiving, Christmas Eve, Christmas Day, New
Years Eve, and New Years Day) are ranked by sta
and determined by mid October. Minor holidays
(Easter, Memorial Day, Fourth of July, and Labor
Day) are ranked by sta and determined by mid
February. In addion to the actual holiday, sta will
be assigned to work the day or days surrounding the
scheduled holiday. Sta with greater than ve years
experience on days and greater than three years
experience on nights may request PTO during winter
holidays. Otherwise, vacaons will not be granted
during the week of a major or minor holiday.
Placed on Call
Sta may be placed on call due to low census or
acuity. Nurses may request to be placed on call
(POC) by subming a rst-o request in Vandy-
works. First-o requests can be placed as early at
1700 on the Saturday before the week of the shi
you are requesng. If a nurse places a rst-o re-
quest within 14 hours of the requested shi, they
must verbally nofy the charge nurse prior to that
shi. If a nurse is granted a rst-o request, then
they will go to the boom of the list for rst-o all
other days of the same week. Sta placed on call
for low holiday census will not be eligible to take
call for their next scheduled holiday unless they
are called in within four hours.
Scheduled on Call
When opmally staed, CVICU will have two nurses
scheduled on call (OCN) at all mes. On call nurses
will be ulized aer available oat pool nurses have
been assigned to the unit. If neither OCN has been
called in during the current schedule, the least sen-
ior sta member will be called in rst.
Floang to Other Units
CVICU nurses may oat to other units depending on
paent census or acuity. Nurses with less than six
months seniority or working their on call shi will
not be oated. Nurses who have not oated to oth-
er units will be oated rst. If all scheduled nurses
have oated, then the determinaon will be made
according to last oat dates.
STANDARDS OF CARE_________________________
The CVICU Standards of Care dene the minimum
amount nursing care that a paent receives while
admied to the CVICU at Vanderbilt. Nurses may
give care that exceeds the pracces outlined in the
Standard of Care. Stepdown Standards of Care may
be applied to paents with transfer orders. A com-
plete copy of the standards of care can be found on
the CVICU website and in Learning Exchange. The
Standards of Care Quick Guide can be found in Box
1.2.
The Standards of Care should also guide
how nurses chart the care they provide.
Use the quick guide to make sure that
all charng is completed accurately each
shi.
PRECEPTOR PEARLS

Back to Table of Contents 11
• COMPLETED Q SHIFT & PRN
Complete Assessment
RASS/CAM ICU scores
Fall Risk Assessment
Braden Skin Assessment
EKG Rhythm, Temporary
Pacemaker Sengs
Alarm Limits
Priority Problems
Plan of Care
• COMPLETED Q4 HOURS & PRN
Cardiac Output, Index and
SVR on PA Cath paents
Transducers Zeroed
Temperature Assessment
Peripheral Pulse Checks
Mouth Care
• COMPLETED Q2 HOURS & PRN
Focused Re-assessment
IABP Unassisted numbers
Pain Assessment
Restraint Assessment
• COMPLETED Q1 HOUR & PRN
Vital Signs
Intake and Output
Device ‘vital signs’
STANDARDS OF
CARE QUICK
GUIDE
BOX 1.2
BEDSIDE REPORT____________________________
Bedside report is expected to be completed during
each shi handover. Bedside report should include
the paent and/or family member and review: code
status, fall risk, restraint use, pernent history and a
full system assessment. Nurses should use this op-
portunity to complete a visual inspecon of all
wounds, incisions, drains or other skin issues, includ-
ing pressure ulcers. Orders should be reviewed with
the o-going shi at this me. Visual inspecon of
all IV infusions, including conrmaon of IV concen-
traons and rates should be reconciled with the or-
ders prior to the o-going nurse leaving. The on-
coming nurse is expected to trace all lines from the
pump to the point of entry and ensure that all lines
are labeled and within the expiraon date.
ESCALATING ISSUES__________________________
Occasionally issues arise that must be addressed. It
is the expectaon of the leadership team that any
paent safety or nursing care issue is addressed pro-
fessionally with the involved sta member(s) at the
me that the concern is noted. If the issue is not
able to be resolved, then the sta member should
escalate the concern to the CSL or RSL on duty. For
provider teams, nurses may ulize the CVICU Urgent
Needs Escalaon Pathway (Figure 1.2) to guide the
escalaon of acute or trending changes that, if le
unaended, could potenally result in paent harm.
Just Culture
Vanderbilt subscribes to the Just Culture philosophy
to improve the working environment for sta and
paent safety. Just Culture promotes professional
accountability between the leadership team and
front-line sta by improving system errors, ulizing
mistakes as learning tools, and promong a blame-
free environment (Boysen, 2013). To this end, sta
are encouraged to report or self-report any errors
that occur so that the leadership team can idenfy
any underlying process issues that may have contri-

Back to Table of Contents 12
-uted to the error. Unless there is reason to suspect
otherwise, the leadership team operates under the
assumpon that any medical errors are non-
malicious in intent and that process improvement or
educaon can prevent future errors of the same
type.
Veritas
Veritas is Vanderbilt’s incident reporng tool and
can be accessed on any clinical work staon. Veritas
provides a system in which interdepartmental lead-
ership teams can collaborate to nd a soluon to
complaints or problems.
CODE ROLES AND RESPONSIBILITES_____________
Codes in the CVICU should be organized with clear
delegaon of roles. If a primary nurse needs to code
a paent, they should promptly press the code
buon and iniate chest compressions. When help
arrives, the charge nurse is responsible for assuring
that all code roles are accounted for. The following
roles should each be assigned to separate nurses:
scribing the documentaon record, managing the
crash cart, and pushing medicaons (Figure 1.1). In
addion to these roles, at least two personnel
should rotate chest compression every two minutes.
Any sta without an ocial code role may be asked
to step outside of the room to facilitate clear lines of
communicaon during the code.
RECOMMENDATION LETTERS__________________
The CVICU Leadership Team will write recommenda-
on leers for graduate school aer the employee
has been a nurse in CVICU for two years.
Figure 1.1
Each code should have a scribe, a nurse pulling drugs from
the crash cart , and a nurse pushing medications in addition
to two compressors.

Back to Table of Contents 13
Figure 1.2
On the medical cardiology service the nurse should address issues with the resident followed by the fellow. If the issue is
not resolved, notify the CCU attending, and, lastly, the CVICU Cardiology Medical Director. On the surgical service the
nurse should escalate concerns through the nurse practitioner or physician assistant, followed by the intensivist fellow,
intensivist and, nally, the CVICU Surgery Medical Director.

Back to Table of Contents 14
R I T I C A L C A R E FO U NDAT IO NS
C
Ca re o f t he Cr i t i ca ll y Il l Ca rd i ac Patie nt
2
Nurses working in cardiovascular intensive care
must have a procient understanding of cardiac
anatomy, physiology and hemodynamics. This chap-
ter will review basic cardiac structures and physiol-
ogy in addion to hemodynamic monitoring devices
and parameters. Relevant pulmonary physiology,
venlator modalies, and ABGs will be reviewed.
CARDIAC STRUCTURAL ANATOMY______________
The normal human heart is a muscular organ that
contains four chambers: two atria in the upper
heart and two ventricles in the lower heart. The
right atrium receives blood from the systemic circu-
laon via the superior and inferior vena cava. The
right atrium then contracts to propel the blood into
the right ventricle. The right ventricle’s subsequent
contracon propels blood through the pulmonary
arteries into the capillary-rich lung beds for oxygen-
aon. On the le side of the heart, the le atrium
receives oxygenated blood from the lungs and pro-
pels this blood into the le ventricle for systemic
circulaon via the aorta.
Valves
Four valves separate the chambers of the heart,
two semilunar valves (aorc and pulmonic) and two
atrioventricular valves (tricuspid and mitral). Appro-
priately named, the pulmonic valve separates the
right ventricle from the lungs while the aorc valve
separates the le ventricle from the aorta. Both
the aorc and pulmonic semilunar valves are rela-
vely small in diameter and receive a high velocity
of blood ejecng from the le and right ventricle.
The atrioventricular valves open during diastole to
allow lling from the atria to the ventricles and
close during systole to prevent retrograde blood
ow into the atria during ventricular ejecon
(Figure 2.2). The atrioventricular valves are held in
posion by the papillary muscles within both ventri-
cles. Valve closure is responsible for the heart
sounds heard on auscultaon with S1 represenng
the closure of the atrioventricular valves and S2
represenng the closure of the semilunar valves.
Figure 2.1 Normal Cardiac Anatomy. Image by
Blausen.com sta (2014). "Medical gallery of Blausen
Medical 2014". WikiJournal of Medicine 1 (2).

Back to Table of Contents 15
Myocardium and Associated Structures
The muscle of the heart, or myocardium, is encased
in the brous pericardial sac. This pericardial sac
holds the heart in posion in the thoracic cavity and
secretes pericardial uid to lubricate the movement
of the heart in the chest. The ventricular myocardi-
um is thicker on the le side of the heart, approxi-
mately 6-11 mm, to generate high pressures need-
ed to circulate blood systemically. Conversely, the
right ventricle is relavely thin-walled and
measures only 2-4mm, generang blood ow to
the low pressure of the pulmonary vasculature
(Figure 2.2).
The Conducon System
Cardiac muscle cells have a resng potenal of
approximately –90mV across their cellular mem-
brane (Sidebotham, McKee, Gillham & Levy,
2007). Specialized pacemaker myocytes spontane-
ously generate electrical impulses to depolarize this
resng potenal, diusing ions across the cell mem-
brane and forcing muscular contracon. The prima-
ry cluster of pacemaker myocytes are housed in
the right atria, known as the Sinoatrial (SA) node.
Understanding valve posion during the
cardiac cycle can inform your clinical
assessment. For example, a systolic
murmur may indicate an incompetent
atrioventricular valve or a stenoc aorc
valve. Both create turbulent blood ow
as the heart ejects blood in systole.
PRECEPTOR PEARLS
Figure 2.2 The Heart in Cross Secon. Images by OpenStax College - Anatomy & Physiology, Connexions Web site. http://
cnx.org/content/col11496/1.6/, Jun 19, 2013., CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=30148207
and By Patrick J. Lynch, medical illustrator - Patrick J. Lynch, medical illustrator, CC BY 2.5, https://
commons.wikimedia.org/w/index.php?curid=1490819

Back to Table of Contents 16
Under normal circumstances, the SA node iniates
and conducts this electricity through intermodal
tracts in the atria to the AV node. The AV Node sub-
sequently slows this conducon to allow the atria
to contract, lling the ventricles with blood prior to
ventricular depolarizaon. Finally, the electrical im-
pulse passes quickly through the bundle of HIS, bi-
furcang into the bilateral bundle branches to al-
low for a swi, coordinated contracon of the bilat-
eral ventricles. It is important to note that any dis-
rupon of blood supply to or the anatomic struc-
tures around the SA or AV node can cause conduc-
on abnormalies. Further discussion regarding
conducon abnormalies and their treatment can
be found in Chapter 6.
Coronary Arteries
The coronary arteries provide oxygenated blood to
the myocardium. Branching from the coronary osa
at the base of the aorta, each coronary artery
branches to perfuse unique areas of the heart
(Figure 2.4). Importantly, the Le Anterior Descend-
ing (LAD) artery supplies approximately 55% of the
blood ow to the le ventricle (LV). For this reason,
the LAD is commonly nicknamed “the widowmak-
er”. This artery branches into the diagonals and
septal perforators, also supplying oxygen to the
ventricular septum. Approximately 20% of the LV
perfusion is supplemented by the Le Circumex
artery and its branches . The Right Coronary artery
supplies blood to the right ventricle (RV) and the
remainder of the LV. Notably, the Right Coronary
artery branches into the acute marginal, posterior
descending and posterior lateral branches to pro-
vide oxygen to the inferior and posterior of the
heart as well as the AV node.
Figure 2.4 Coronary Arteries, Anterior View. Image by Tvanbr
- Own work, Public Domain, https://commons.wikimedia.org/
w/index.php?curid=11645241
Figure 2.3 The Conduction System. Image by Cypressvine -
Own work, CC BY-SA 4.0, https://commons.wikimedia.org/
w/index.php?curid=80381713

Back to Table of Contents 17
Cardiac Physiology and the Cardiac Cycle________
The cardiac cycle has two primary phases: systole
and diastole. The closure of the atrioventricular
valves, heard as S1 on auscultaon, marks the onset
of cardiac systole. Electrical conducon through the
ventricle prompts ventricular contracon and in-
creases the pressure within the ventricles, known
as isovolumetric contracon. As this pressure over-
comes aorc and pulmonary artery pressure, the
aorc and pulmonary valves open against the favor-
able pressure gradient and allow for a rapid ejec-
on of blood. Systolic blood pressure, therefore, is
an indirect indicator of cardiac output.
Diastole, or ventricular lling, begins with the clo-
sure of the semilunar valves as the pressure in the
ventricle falls below the resng aorc pressure. The
closing of the semi lunar valves is heard as S2 on
auscultaon. Simultaneously, the low pressure of
the ventricle in diastole allows the atrioventricular
valves to open against a favorable gradient, allow-
ing for rapid inow of blood from the atria. Ventric-
ular relaxaon promotes blood ow into the coro-
nary arteries, perfusing the myocardium during di-
astole. At a normal resng heart rate, diastole rep-
resents two-thirds of the cardiac cycle, promong
adequate oxygenaon and resng of the mycocar-
dium.
Figure 2.5 Wigger’s Diagram. Public Domain.
Systole commences with the closure of the atrioventricular valves, heard as S1 on auscultation. Moving against a
favorable pressure gradient, the aortic and pulmonary valves opens to allow for a rapid outow of blood as the
ventricles contract. Diastole commences with the closure of the aortic and pulmonary valves. Simultaneously, the
atrioventricular valves reopen to allow for rapid ventricular lling.

Back to Table of Contents 18
Determinants of Adequate Cardiac Output_______
Cardiac output is the amount of blood that the
heart is able to pump in one minute. Measured in
liters/minute, cardiac output quanes the health
of the heart muscle and provides insight into other
physiologic mechanisms contribung to the global
paent picture. At the most basic level, cardiac out-
put is determined by stroke volume and heart rate.
Stroke volume is dened as the volume ejected
with each heart beat (Sidebotham, McKee, Gillham
& Levy, 2007). It is important to note, however, that
many factors impact stroke volume, chief of which
include the following three physiologic concepts:
preload, aerload, and contraclity.
Preload
Funconally, preload represents the volume of
blood returning to the heart during diastole. Thus,
preload is generally considered a measurement of
end diastolic volume and is commonly referred to
as lling pressure. Without adequate blood to ll
the ventricles, the heart cannot generate an ade-
quate stroke volume. Preload also indirectly im-
pacts the contraclity of the heart, as described by
Starling’s Law of the Heart. This law draws denotes
that increased volume and ventricular stretch in-
creases the force of contracon from the ventricles.
However, the force of contracon does have limita-
ons and parcularly high ventricular volumes may
decrease contraclity of the heart. For this reason,
it is clinically important to assess and opmize uid
status in the crically ill cardiac paent.
Aerload
Aerload represents the resistance against which
the ventricles must eject and is also representave
of ventricular wall stress. Most commonly, this re-
sistance is secondary to systemic vascular changes
and complex physiologic mechanisms. For example,
a paent who is chronically hypertensive is in a
chronic high aerload state. Conversely, many cri-
cally ill paents may decrease their aerload as a in
stages of sepc or anaphylacc shock. The diastolic
blood pressure is considered to be an indirect
measurement of systemic vascular resistance.
Contraclity
Contraclity represents the ability of the heart to
contract, independent of preload and aerload.
This is essenally a measure of the health of the
myocardium. Unfortunately, there is no direct he-
modynamic measure of contraclity. However,
echocardiography, paent history, and assessment
of ejecon fracon (EF) may inform the conclusion
that the heart is not contracng eecvely.
Hemodynamic Monitoring____________________
Hemodynamic monitoring ulizes invasive lines to
provide and array of data regarding the volume,
pressure and ow of blood throughout the body to
guide treatment decisions. However, the ulity of
these lines is largely based on the accuracy of the
measurement. The following secons will review
the types of hemodynamic monitoring provided in
the CVICU with emphasis on nursing intervenons
to assure the accuracy of these measurements.
Arterial Blood Pressure Monitoring
Arterial lines are lines placed in the radial, brachial
Figure 2.6 A Normal Arterial Waveform
A
B

Back to Table of Contents 19
or femoral arteries that transduce the mechanical
pressure changes of systole and diastole into a
pressure changes during the cardiac cycle to the
Phillips monitor. Arterial lines are the gold standard
of blood pressure monitoring, and provide connu-
ous, real-me data. A normal arterial waveform
should have a demonstrable systolic pressure read-
ing (Figure 2.6, A) and a visible dicroc notch
(Figure 2.6, B), represenng the closure of the aor-
c valve.
As with all IV lines transducing pressure, an arterial
line must be transduced using non-compressible
tubing aached to a transducer (Figure 2.7). To en-
sure accuracy, this transducer is leveled to the
heart at the phlebostac axis (5th intercostal space,
mid-axillary line) and zeroed to atmospheric pres-
sure.
Central Venous Pressure
Central Venous Pressure (CVP) can be measured by
transducing an internal jugular (IJ) or subclavian
line. This pressure reects the pressure in the IJ or
le atria and is largely a reecon of preload or vol-
ume status. It is important to note that, similar to
an arterial line, the CVP waveform reects mechani-
cal changes in the vasculature. During atrial systole,
the pressure increase in the atria reects posive
pressure toward the IJ catheter (Figure 2.8, A). tri-
cuspid valve closes, a small notch can be seen
(Figure 2.8, C). Finally, as the atria lls again during
atrial diastole, another inux of pressure can be
noted (Figure 2.8, V). Because the pressure trans-
duced is from the venous vasculature, the pressure
gradients between systole and diastole are much
smaller. For this reason, a mean pressure gradient
is considered accurate. Normal CVP ranges from 2-8
mm/Hg. As with all transduced pressures, accuracy
is dependent upon the nurse leveling the transduc-
er to the phlebostac axis and zeroing the pressure
to atmosphere.
Figure 2.7 A schematic of a typical pressure transducer. From
Kruse, J.A., Fink, M.P., Carlson, R.W. [Eds.].
[2003]. Saunders manual of critical care
medicine. Philadelphia: Saunders. Used with permission (El
Sevier, 2019)
Figure 2.8 Central Venous Pressure Waveform. From
Wiegand, D.L. [Ed.]. [2017]. AACN procedure manual for
high acuity, progressive, and critical care [7th ed.]. St.
Louis: Elsevier . Used with Permission (El Sevier, 2019)

Back to Table of Contents 20
Pulmonary Artery Catheters
Pulmonary artery (PA) catheters are catheters in-
serted through the IJ and advanced so that the dis-
tal p rests in the pulmonary artery. Various ports
open along the trajectory of the PA catheter, allow-
ing clinicians to transduce data from mulple places
in the heart. PA catheters connuously transduce a
CVP from the right atrium via a proximal port as
well as pressures from the pulmonary artery via
a distal port. Because the pulmonary arteries
are in the pulmonary circulaon, these pres-
sures are much lower than the systemic pres-
sures. Nonetheless, the pulmonary artery loca-
on is distal to the pulmonic valve, and pres-
sures transduced within the pulmonary artery
should exhibit an arterial waveform paern
with disnct systole, diastole and dicroc notch.
Normal PA systolic pressures should read 20-30
mmHg. Normal PA diastolic pressures should read 5
-10 mmHg. Elevated PA pressures may indicate es-
senal pulmonary hypertension, embolus or other
pathology increasing the vascular resistance in the
lung beds.
PA catheters are “oated” into the pulmonary ar-
tery by inang a balloon on the p of the catheter
that acts as a sail, pulling the catheter further into
the heart. As the catheter is inially inserted, the
nurse should noce a CVP waveform from the distal
PA catheter port since the catheter is sing within
the right atrium (Figure 2.10, A). As the provider
advances the catheter, the waveform will demon-
strate a dynamic ventricular waveform (Figure 2.10
B). Finally, as the catheter advances past the pul-
monic valve, the waveform assumes the character-
isc arterial waveform and reects the pressures of
the pulmonary artery (Figure 2.10 C). On inseron,
the provider will connue to advance the catheter
unl the balloon wedges in the pulmonary artery
(Figure 2.10, D. Since there are no valves between
the pulmonary artery and the le atrium, the
wedge pressure is considered to be representave
of le atrial lling pressures. Nurses should note
that the wedge pressure assumes a waveform
Figure 2.9 PA Catheter. Image by Chikumaya, Drawn
with Inkscape 0.43 - Own work, CC BY-SA 3.0, https://
commons.wikimedia.org/w/index.php?curid=817738
It is important to assess the character of
the PA waveform. If the PA catheter
becomes wedged unintenonally, it could
block blood ow within the heart and
cause pulmonary infarcon. A dampened
or wedged waveforms from the PA
catheter should be invesgated
immediately
PRECEPTOR PEARLS

Back to Table of Contents 21
similar to that of a CVP but with higher mean pres-
sures. Average pulmonary capillary wedge pressure
(PCWP) is 4-12 mmHg. Increased PCWP may indi-
cate uid overload, LV failure, mitral stenosis or mi-
tral regurgitaon. Low PCWP may indicate
hypovolemia or the use of venodilators (Alspach,
2006). Wedge pressures are measured upon inser-
on and PRN by CCU fellows or cardiac surgery
APRN
Cardiac Output and Index
PA catheters also allow for the direct measurement
of cardiac output. While there are many methods
to calculate cardiac output, CVICU most commonly
uses a thermodiluonal PA catheter. Using this
method, 10 ml of room temperature normal saline
is rapidly infused through the proximal (CVP) port
of the catheter. The distal end of the catheter then
measures the change in temperature, and uses this
informaon to calculate the rate of blood ow
through the heart in one minute. The average cardi-
ac output ranges from 4-8 L/min, depending on
body size and physiologic demand. To simplify the
evaluaon of this informaon, cardiac output can
A
B
D
C
Figure 2.10 Normal Waveforms while oating a pulmonary artery catheter. From Urden, L.D., Stacy, K.M., Lough, M.E.
[Eds.]. [2018]. Critical care nursing: Diagnosis and management [8th ed.]. Maryland Heights, MO: Elsevier.

Back to Table of Contents 22
be normalized for a paent’s body surface area
(BSA). This number, the cardiac index (CI), normally
ranges from 2.5-4 L/min/m
2
. In the CVICU, a CI less
than 2 L/min/m
2
is a reportable value to the provid-
er team. A decrease in cardiac output is likely an
indicaon of decreased preload (volume), increased
aerload (resistance) or decreased contraclity.
Paents in moderate to severe sepc shock may
exhibit dramac decreases in aerload that subse-
quently increase the total amount of cardiac out-
put.
Systemic and Pulmonary Vascular Resistance
With the addion of a cardiac output, systemic and
pulmonary vascular resistance can be calculated.
Systemic vascular resistance (SVR) uses the mean
arterial pressure (MAP), CVP, and cardiac output
(CO) to esmate the total resistance to ejecon
during systole. Therefore, the SVR is a direct reec-
on of systemic aerload. Conversely, the pulmo-
nary vascular resistance (PVR) can be calculated us-
ing the mean PA pressure, PCWP and cardiac out-
put. This value uniquely reects the aerload expe-
rienced by the right side of the heart during systole.
Normal SVR ranges from 800-1400 dynes/sec/cm
-5
and normal PVR ranges from 50-250 dynes/sec/cm
-
5
. Both SVR and PVR provide tremendous value in
dierenang types of heart failure, types of shock
and determining the course of treatment in mixed
shock.
MECHANICAL VENTILATION___________________
Paents may need to be mechanically venlated for
a variety of reasons, including but not limited to:
inability to protect their airway, respiratory distress
or arrest, sedaon, or severe oxygenaon issues. At
Vanderbilt, the ICU fellow, aending or respiratory
therapist (RT) are the only personnel allowed by
policy to make venlator changes. Nonetheless,
nursing sta must maintain a basic knowledge of
venlator modalies, ancipate venlator changes
related to the paent’s clinical picture, and trouble-
shoot common alarms.
In a healthy individual, respiratory rate and depth
are determined by the autonomic nervous system.
A negave intrathoracic pressure drives inhalaon
as the diaphragm lowers with each breath. In the
mechanically venlated paent, however, respira-
tory rate and depth care controlled manually. Res-
piraon is instead driven by posive pressure via
the venlator. Venlator modalies are named by
the specic component of venlaon manipulated
in each mode, classically volume control or pressure
control modes. Venlator parameters are reviewed
in Box 2.1. Venlator modes can be compared in
Box 2.2.
Assist Control Venlaon
In assist control (AC) venlaon, a set number of
breaths are delivered in a given amount of me.
This modality can then be further specied as vol-
ume control (VC) or pressure control (PC). In vol-
ume control venlaon, the dal volume of each
respiraon is set, but the pressure required to de-
liver this volume will vary with each breath. By con-
trast, pressure control venlaon sets the amount
of posive pressure that will be used to deliver
each breath, allowing the volume to vary. In both
Aer PA catheter inseron, wedge
pressures should only be obtained on
medical cardiology paents with a
physician order. Due to the risk of PA
rupture and infrequency of use, our
standards of care spulate that only
physicians or advanced pracce providers
wedge paents.
PRECEPTOR PEARLS

Back to Table of Contents 23
modalies, respiratory rate, posive end expiratory
pressure (PEEP), and amount of oxygen (FiO2) deliv-
ered are set. If the paent aempts to breath in be-
tween these breaths, the venlator will assist the
paent with these breaths to deliver the designated
volume or pressure. This may unintenonally cause
the paent to hypervenlate, and nurses should be
careful to note the dierence between the set num-
ber of venlator breaths and the delivered number
of venlator breaths.
Synchronized Intermient Mandatory Venlaon
In a synchronized intermient mandatory venla-
on (SIMV), the venlator also delivers a set num-
ber of breaths during a given period of me using
pressure or volume control. However, in this modal-
ity, the paent may breathe spontaneously between
the venlator-mandated breaths. Importantly, the
venlator will aempt to synchronize mandatory
breaths with spontaneous paent eort, making
this mode of venlaon more comfortable for the
paent and decreasing the risk of hypervenlaon
(Parillo & Delinger, 2014). This mode of venlaon
requires more paent eort than assist control ven-
laon. The Vanderbilt CVICU most commonly uses
SIMV with a dual control mode of venlaon known
as pressure-regulated volume control (PRVC). In
PRVC, the volume for each respiraon is set, but the
pressure required to deliver this volume is limited.
Pressure Support
Pressure support (PS) mode of venlaon is consid-
ered to be a weaning mode of venlaon. In pres-
sure support, the rate, dal volume and inspiratory
pressure are not set, requiring the paent to iniate
their own breaths and maintain their own respirato-
ry rate.
Alarms and Troubleshoong
Common venlator alarms include: PAW high, High
Respiratory Rate, and Regulaon Pressure Limited.
An airway pressure high (PAW High) alarm indicates
increased airway pressure caused by blockage in the
venlator circuit. Common causes include: paent
coughing or bing the endotracheal tube (ETT), pa-
ent needing to be suconed, the Heat Moisture
Exchanger (HME) needing to be changed, or
crimped tubing. Nurses should assess the paent
and the venlator tubing to address this alarm, suc-
oning the paent as needed. If the HME appears
saturated, nurses may change the HME on the ven-
lator circuit.
High respiratory rate alarms indicate the that respir-
atory rate has exceeded the alarm limit. This may be
caused by pain or anxiety requiring increased seda-
on or by paent decompensaon. Nurses should
address the root cause of the increased respiratory
rate as warranted by the paent picture. Nurses
should also assess whether the respiratory rate
alarm is appropriate for the paent and nofy res-
piratory if the alarm limit should be reconsidered.
FiO2 Fracon of inspired oxygen. The amount
of oxygen delivered to the paent.
PEEP Posive End Expiratory Pressure. The
amount of posive pressure connuously
delivered to the paent to keep the alve-
oli open.
PIP Peak Inspiratory Pressure. The maximum
amount of pressure needed to inate the
desired volume of air. PEEP + PC sengs
= PIP
Respiratory
Rate
The number of respiraons in one mi-
nute.
Tidal Volume The volume of air inslled with each res-
piraon. Inspiratory volume should equal
expiratory volume.
Minute Venla-
on
Tidal volume x respiratory rate. The total
amount of air venlated in one minute.
Measured in L/min.
BOX 2.1

Back to Table of Contents 24
Mode Denion When Used Key Clinical Points
SIMV
Synchronized
Intermient
Mandatory Ven-
laon
Delivers set number of breaths at a set
volume per minute synchronized to go
with paent’s breathing paern
Gives more control
Most post-op paents are
on this and PRVC, allows
paent to do some of the
work. Weaning mode.
Paent can take own breaths but
the set dal volume is not deliv-
ered with those breaths. Pres-
sure support is used for those
breaths
PRVC
Pressure Regu-
lated Volume
Control
Control mode of venlaon with a set
rate, set dal volume, and set PEEP. The
venlator regulates how much pressure
control needed by the set dal volume.
Does not aid spontaneous breaths.
Used in paents with less
compliant lungs such as
ARDs and post op paents.
Combined with SIMV. Al-
ternates modes.
By itself, PRVC is not a
weaning mode.
Control is taken back over by the
venlator, so paent cannot ini-
ate own breaths as easily, thus
can be anxiety producing mode.
CPAP and pres-
sure support
Connuous Pos-
ive Airway
Pressure
Posive pressure exerted on lungs. Con-
stant pressure through inspiratory and
expiratory cycle. Pressure support added
to help aid in keeping lungs open during
inspiraon
Used before extubaon to
see if paent can breath on
own. Paent creates own
dal volume and rate.
Paent must be able to sponta-
neously breathe.
Volume Control
(also known as
Assist control)
Control venlaon with set rat and set
dal volume. Paent can iniate a breath
and venlator will give a breath
Used for paent with low
spontaneous dal volumes.
Not weaning mode.
Can decrease cardiac output.
Does not have pressure support.
Bi-Vent Inverse expiratory and inspiratory rate.
Prolongs inspiratory me with short expir-
atory me.
ARDS, decreased Sa02 and
increased peak pressures.
Unable to venlate.
Very uncomfortable! Ensure ade-
quate sedaon.
VDR
Volumetric
Diusive Respi-
rator
High frequency venlator. High rate with
low dal volumes. Oxygenaon occurs
through diusion. Internal percussion.
ARDs, low Sa02 with in-
creased PIP, unable to ven-
late in other modes.
N/A
BOX 2.2
Venlator Modes
A regulaon pressure limited alarm occurs in PRVC
mode when the PIP becomes too high to allow the
venlator to deliver the set dal volume. This may
indicate that the paent’s lung compliance is de-
compensang or that the paent is ghng the ven-
lator. Nurses should asses their paent to deter-
mine whether the paent appears to be in pain and
ascertain whether increased sedaon is warranted.
If no discernable cause can be determined, the
nurse should nofy respiratory and the care team.
Venlator Weaning
Spontaneous awakening trials (SATs) and spontane-
ous breathing trials (SBTs) should be conducted dai-
ly on venlated paents in collaboraon with the
interdisciplinary care team. Prior to conducng an
SAT, the nurse should complete the SAT safety
screen (Box 2.3). To conduct an SAT, the nurse

Back to Table of Contents 25
should pause sedaon and analgesia infusions unl
an adequate neurological exam can be obtained. If
the paent is able to follow commands with seda-
on weaning, then the nurse should conduct an SBT
safety screen and contact the care team for approv-
al to begin an SBT. If the team approves to paent
to parcipate in an SBT, the RT will place the venla-
tor into pressure support on minimal venlator
sengs. However, if the paent experiences anxie-
ty, agitaon or pain that cannot be managed by prn
medicaon, respiratory distress or any acute cardiac
rhythm changes, the nurse should resume connu-
ous sedaon and contact the RT to place the paent
back on full venlator support. In the CVICU, it is our
goal to extubate paents within six hours of cardiac
surgery unless their clinical exam warrants other-
wise. Once the paent achieves hemodynamic sta-
bility aer surgery, the nurse should collaborate
with the cardiac surgery team to determine when it
will be appropriate to wean sedaon for an SAT.
ARTERIAL BLOOD GAS MONITORING____________
Arterial blood gases (ABGs) measure the content of
oxygen (PO2) and carbon dioxide (CO2) in dissolved
in a paent’s blood. Values generated through an
ABG sample will oer insight into a paent’s acid-
base balance, determine the source of imbalances,
and guide treatment changes in the crically ill pa-
ent. To maintain normal cellular metabolism, a
narrow pH range of 7.35-7.45 must be maintained.
A pH of less than 7.35 is considered acidoc while a
pH greater than 7.45 is considered alkaloc. Under
normal circumstances, the human body maintains
homeostasis by regulang HCO3– through the kid-
neys and CO2 via the lungs (Figure 2.12). If the pri-
mary abnormality in the ABG is in the paCO2, then
the disturbance is considered respiratory in nature.
If the primary abnormality in the ABG is the HCO3-,
then the disturbance is considered metabolic in na-
ture. To interpret an ABG, the nurse should rst de-
termine whether the pH is normal, acidoc
Figure 2.12 Acid-Base Imbalance. From Betts, J.G, Young,
K.A., Wise, J.A., Johnson, B., Poe, B, Kruse, D.H….DeSaix,
P. Anatomy and Physiology. Houston, Tx: OpenStaxx
SAT Safety Screen
No acve seizures No agitaon
No alcohol withdrawal No paralycs
Normal Intracranial Pressure
No myocardial ischemia
SBT Safety Screen
No agitaon Inspiratory Eorts
Oxygen Saturaon >88%
Fio2 < 50%
PEEP < 7.5 cm H2O
No vasopressor use
BOX 2.3

Back to Table of Contents 26
or alkaloc. The nurse should then determine
whether the paCO2 and HCO3– are within normal
ranges (Box 2.4).
Respiratory Acidosis and Alkalosis
Respiratory acidosis is caused by any condion that
induces hypovenlaon, thus elevang a paent’s
CO2. This may include primary lung diseases, seda-
on impeding a paent’s intrinsic respiratory drive,
brain injuries, neuromuscular disorders, pneumo-
thorax, and trauma impeding breathing mechanics,
or incorrect venlator sengs (American Associa-
on of Crical Care Nurses, 2018). Conversely, res-
piratory alkalosis is caused by any condion that
induces hypervenlaon such as hypoxemia, sepsis,
or even anxiety (American Associaon of Crical
Care Nurses, 2018). In mechanically venlated pa-
ents, nurses can expect providers to increase or
decrease a paent’s minute venlaon by manipu-
lang dal volume and respiratory rate to migate
an acidosis or alkalosis of respiratory origin. An in-
crease in minute venlaon will decrease pH. A de-
crease in minute venlaon will increase pH.
Metabolic Acidosis and Alkalosis
A decrease in HCO3– causes metabolic acidosis.
Causes most commonly include diabec ketoacido-
sis, lacc acidosis, and renal failure (American Asso-
ciaon of Crical Care Nurses, 2018). Metabolic al-
kalosis is characterized by an increase in HCO3– lev-
els and is most commonly caused by diuresis, vom-
ing, or potassium deciency. Notably, massive
transfusion may cause metabolic alkalosis due to
citrate in the preserved blood products.
Compensaon
Paents may parally or completely compensate
for acid-base abnormalies. Paents who have
compensated will demonstrate a normal or near-
normal acid-base balance. To compensate for res-
piratory abnormalies, the kidneys will either ex-
crete or reabsorb addional HCO3-. Compensaon
for respiratory acidosis, therefore, is characterized
by a normal or near normal pH with abnormally in-
creased HCO3 and CO2. Compensaon for respira-
tory alkalosis is characterized by a normal or near-
normal pH with decreased HCO3 and CO2. Renal
compensaon for respiratory abnormalies may
take several days.
To compensate for metabolic abnormalies, a pa-
ent may regulate breathing to retain or remove
CO2. For metabolic acidosis, a paent may increase
their respiratory rate and depth to decrease the
amount of CO2 dissolved in the blood. Conversely,
a paent with metabolic alkalosis may decrease
their respiratory rate and depth to retain CO2. Res-
piratory compensaon occurs faster than metabolic
compensaon, taking only a few hours to compen-
sate for abnormalies.•
METRIC NORMAL ABG NORMAL VBG
pH 7.35-7.45 7.31-7.41
P02 80-100 mm Hg 35-40 mm Hg
SaO2 > 94% 70-75%
paCO2 35-45 mm Hg 41-51 mm Hg
HCO3- 22-26 mEq/L 22-26 mEq/L
Base Excess -2 to +2 -2 to +2
BOX 2.4 NORMAL ABG VALUES

Back to Table of Contents 27
Use the knowledge gained in this chapter and the following scenario to answer the quesons below. When you
are ready, check you answers on p. 69.
You are caring for a paent admied to the CVICU yesterday for cardiogenic shock. The paent is
intubated on the following venlator sengs: SIMV/PRVC; FiO2 60%; Rate 12; TV 450; PEEP 8. The
paent has a PA catheter inserted to a depth of 48 cm with the following data populated on the
Phillips monitor: CVP 18 mmHg; PA 45/25, Wedge pressure 24 mmHg.
1. Based on the data presented, you assess that the paent is:
A. Volume overloaded
B. Euvolemic
C. Volume depleted
2. Aer compleng your 0800 thermodiluonal cardiac output measurement, you note that the
paent’s cardiac index is 1.7 . Based on this informaon, you r rst acon is to:
A. Connue to monitor the paent . This index is expected for a paent in shock.
B. Nofy the provider because the cardiac index is below the threshold for reportable values.
C. Review the paent’s PVR and SVR to evaluate the type of heart failure the paent exhibits.
3. You send an ABG and note the following values: pH 7.30; PO2 96%; PaCO2 50; HCO3-24. Based on
this informaon, you expect the provider to make what venlator change?
A. Increase the venlaon rate to increase the minute volume
B. Decrease the venlaon rate to decrease the minute volume
C. Increase the venlaon rate to decrease the minute volume
A P P LY Y O U R K N O W L E D G E : CLINICAL CASE STUDY

Back to Table of Contents 28
R I T I C A L C A R E P h a r ma colo gy
C
3
Crical care nurses are responsible for understand-
ing the therapeuc eects of the drugs that they
administer as well as potenal adverse reacons
and key points of paent educaon. This chapter
will review common classes of drugs ulized in the
CVICU to care for the acutely ill cardiac paent. The
list of drugs included in this chapter is not an ex-
hausve list of drugs administered in the crical
care environment, however. Nurses should use ap-
propriate clinical resources available at the bedside,
such as Lexicomp, to inquire about addional or-
dered medicaons prior to administraon.
VASOPRESSORS & INOTROPES________________
Vasopressors and inotropes agents of choice to
manage shock states and hemodynamic instability
in the post cardiac surgery paent. These agents
augment intrinsic cardiac output by acng on the
adrenergic receptors in the sympathec nervous
system to either constrict the peripheral vascula-
ture or augment cardiac contraclity and heart
rate. Specic adrenergic receptors and their physio-
logic funconality can be found in Box 3.1. A com-
parison of hemodynamic consequences of select
vasopressors and inotropes can be found in Box 3.2.
Dopamine
Dopamine augments cardiac output and MAP by
increasing cardiac contraclity, heart rate, and SVR.
Doses typically range from 2 to 20 mcg/kg/min. The
hemodynamic eects of dopamine are dose-
dependent, with increased inotropy between 4 and
10 mcg/kg/min and increased alpha acvity at dos-
es greater than 10 mcg/kg/min (Hardin & Kaplow,
2020). This drug is most commonly trated by pro-
vider order. In recent years, dopamine has fallen
out of favor due to its increased risk of tach-
yarrhythmias at higher doses. Due to the risk of ex-
travasaon, dopamine should be administered
through a central line.
Epinephrine
Epinephrine is a potent inotrope and vasopressor,
with parcularly vigorous inotropic properes at
higher doses. Connuous infusions may be iniated
at 1 mcg/min and trated by 1 mcg/min every 2
minutes unl desired eect or a maximum dose of
10 mcg/min is achieved. A provider order is re-
quired to trate epinephrine. Due to risk of extrav-
asaon, epinephrine should be administered
through a central line.
RECEPTOR ANATOMICAL
LOCATION
PHYSIOLOGIC
FUNCTIONALITY
Beta 1 Heart Contraclity
Beta 2 Lungs/
Vasculature
Vasodilaon
Alpha 1 Vasculature Vasoconstricon
Vasopressin Vasculature Vasoconstricon
BOX 3.1 ADRENERGIC RECEPTORS

Back to Table of Contents 29
Norepinephrine
Norepinephrine is a both a vasopressor and an in-
otrope. The drug demonstrates much higher anity
for alpha receptors than beta 1 receptors, thus it is
most commonly ulized for post-operave hypo-
tension when a paent’s SVR is low. Norepineph-
rine is also the inial vasopressor of choice in sepc
shock (Rhodes, 2017). Norepinephrine is trated by
nursing within the parameters outlined in the eStar
order sets. The drug is iniated at 2-4 mcg/min and
trated by 1-4 mcg/minute every 2 minutes to
achieve desired blood pressure goal. The maximum
dose is 30 mcg/min, although providers should be
noed for doses greater than 10 mcg/min. Due to
the risk of extravasion, norepinephrine should be
administered via central line.
Phenylephrine
Phenylephrine is a vasopressor with a rapid onset
and a short half-life. It is used to treat post-
operave hypotension in paents with decreased
SVR and may be parcularly useful in paents with
tachyarrhythmias due to its lack of B1 smulaon.
Connuous infusion should be started at 20 mcg/
min and trated by 20 mcg/min to desired dose.
The maximum dose is 240 mcg/min. Phenylephrine
may also be given as a 100-500 mcg bolus every 15
minutes and may be used as adjuncve treatment
during rapid sequence intubaon for propofol-
related hypotension.
Vasopressin
Vasopressin is a pepde hormone that acts on
vasopressin receptors to increase SVR. While
vasopressin has a variety of clinical uses, it is
most commonly used in CVICU to manage post-
operave hypotension aer reaching maximum
doses of norepinephrine. The drug is iniated at
0.04 units/min and is not trated. Rarely, pro-
viders may increase vasopressin dose to 0.06
units/min for persistent hypotension.
VASODILATORS_& ANTIHYPERTENSIVES_________
Vasodilators are considered the preferred agents to
treat postoperave hypertension following cardiac
surgery, reducing blood pressure and preserving
cardiac output by reducing aerload (Hardin &
Kaplow, 2020). However, due to increased cost of
commonly used vasodilators, providers will fre-
quently order IV calcium channel blockers and, less
commonly, beta blockers in place of IV vasodilators
immediately following cardiac surgery. Beta block-
ers are the preferred agent following certain cardio-
thoracic procedures such as aneurism repairs due
to their eect on decreasing heart rate, blood pres-
sure and, cardiac output concurrently. Hemody-
namic consequences of select vasodilatory agents
can be found in Box 3.3.
Esmolol
Esmolol is a short-acng β1 selecve beta blocker
used for postoperave hypertension and tach-
yarrhythmias. Esmolol is also the drug of choice for
vascular paents receiving IV beta blockers. Inial
dosing begins at 50 mcg/kg/min and may be trat-
ed by 50 mcg/kg/min every 2-10 minutes to a max
dose 300 mcg/kg/min to achieve therapeuc goals.
Side eects of esmolol include bradycardia and hy-
potension.
Although beta blockers are a cornerstone
of heart failure management, beta
blockers should avoided in paents
demonstrang decompensated heart
failure or cardiogenic shock. These
paents rely on increased heart rate to
maintain their cardiac output and may
become hemodynamically unstable with
beta blocker administraon.
PRECEPTOR PEARLS

Back to Table of Contents 30
For these reasons, esmolol should not be used in pa-
ents in cardiogenic shock or in a second or third
degree hear block (Hardin & Kaplow, 2020). Nurses
should also exercise cauon administering esmolol
concurrently with a calcium channel blocker. Con-
current administraon may exacerbate hypotension
and bradycardia in some paents.
Labetalol
Labetalol is a non-selecve beta-blocker that also
demonstrates inhibitory eects on alpha receptors.
For this reason, labetalol is generally considered to
be the preferred IV beta blocker for blood pressure
control and may be used as a terary agent follow-
ing nicardipine and hydralazine for post-operave
hypertension. Labetalol may be iniated as either a
10 or 20 mg IV push over 2 minutes, given at 10 mi-
nute intervals unl therapeuc target is reached.
Labetalol may also be given as a connuous infusion
starng at 0.5 to 2 mg/minute with a maximum dose
of 10 mg/minute. A total maximum dose of 300
mg/24 hours must be considered during administra-
on. As with esmolol, labetalol should not be used in
paents exhibing decompensated heart failure or
bradyarrhythmia (Hardin & Kaplow, 2020). Due to
the non-selecve beta acvity of labetalol, paent
may be at increased risk of bronchospasm and
should be avoided in paents with bronchospasc
disease.
Hydralazine
Hydralazine is an IV and PO vasodilator. The IV form
of the drug is commonly used as a second line agent
to manage post-operave hypertension not respon-
sive to calcium channel blockers or other IV vasodila-
tors. Hydralazine may be ordered post-operavely as
a 10-20 mg IV push every four to six hours PRN.
However, due to the risk of reex tachycardia, low-
dose hydralazine is recommended. Hydralazine PO
may also be given to paents in heart failure with
reduced ejecon fracon (HFrEF) who cannot toler-
ate ACE or ARB therapy (Colucci, 2018).
Nicardipine
Nicardipine is an IV calcium channel blocker that
causes arterial vasodilaon in peripheral and coro-
nary vasculature (Krako, 2017). The drug is com-
monly used as a rst-line agent for postoperave
hypertension due to the cost of other preferred vas-
odilators such as nitroprusside. Nicardipine IV may
be iniated at 5 mg/hr and trated by 2.5-5mg/hr
every 5 minutes to a maximum dose of 15 mg/hr.
Although nicardipine has minimal chronotropic
eect, reex tachycardia has been reported in the
literature. Nicardipine demonstrates mild negave
inotropic eects and should be used with cauon in
paents with heart failure with le ventricular dys-
funcon (Yancy et al., 2013).
Nitroglycerin
Nitroglycerin is an arterial and venous vasodilator
used as an anhypertensive agent. Due to its eects
on both the arterial and venous vasculature, nitro-
glycerin is eecve at reducing preload, systemic
aerload, and pulmonary arterial pressures. Im-
portantly, nitroglycerin also has eect on dilang the
coronary arteries, making it an excellent drug to
treat myocardial ischemia and funcon as an an-
anginal agent. The drug is available in mulple dos-
age forms, including IV, oral tablet, sublingual solu-
on, and transdermal patch or ointment. IV nitro-
glycerin is iniated at 5 mcg/min and trated by 5
mcg/min every 5 minutes to desired eect. Maxi-
mum dosage of IV nitroglycerin is 400 mcg/min. Hy-
potension may occur in some paents, parcularly
those paents who are hypovolemic. Headache has
also been reported in higher doses.
Nitroprusside
Nitroprusside is an powerful arterial and venous vas-
odilator used to reduce aerload with secondary
eects decreasing wedge pressure and CVP.
Back to Table of Contents 31
Compared to nitroglycerin, nitroprusside has more
arterial potency. Dosing is iniated at 0.1 mcg/kg/
min and increased by 0.1-0.5 mcg/kg/min every 2-5
minutes unl desired eect is achieved.
INODILATORS_______________________________
Milrinone and dobutamine are two drugs that in-
crease inotropy while minimizing vasoconstricon
or promong vasodilaon. The combined inotropic
and vasodilatory eect of milrinone and dobuta-
mine make them exceponal drugs to treat end
stage heart failure and cardiogenic shock.
Milrinone
Milrinone is a phosphodiesterase inhibitor used to
treat low cardiac output states by increasing the
amount of available calcium in myocardial ion chan-
nels, thus increasing myocardial contraclity. By the
same mechanism, milrinone decreases intracellular
calcium concentraons in the peripheral vascula-
ture, thus allowing for venous and arterial vasodila-
on (Hardin & Kaplow, 2020). Milrinone is started
as a connuous infusion at 0.375 mcg/kg/min and
trated by provider order by increments of 0.125
mcg/kg/min. Notably, milrinone has a relavely
long half-life of two to three hours, causing the
drug’s eect to linger for a prolonged period of
me aer it has been disconnued. The vasodilato-
ry properes of the drug contribute to drug-related
hypotension in some paents.
Dobutamine
Dobutamine is a β1 agonist that, unlike other β1
agonists, has minimal eect on α1, minimizing vaso-
constricve properes and decreasing compensato-
ry vasoconstricon as cardiac output improves. Do-
butamine is iniated at 2.5-5 mcg/kg/min and -
trated by provider order to achieve desired eect.
Maximum dose is 20 mcg/kg/min, although higher
doses have anecdotally been reported. Similar to
other β1 agonists, dobutamine demonstrates an
increased risk of tachyarrhythmias but at a lower
incidence than dopamine. Hypovolemia may exac-
erbate drug-related hypotension and paents
should be adequately uid resuscitated prior to or
concurrent with administraon.
ANTI ARRHTHYMICS & HEART RATE CONTROL____
Amiodarone
Amiodarone is an anarrhythmic given in the
seng of atrial brillaon , ventricular brillaon,
or unstable ventricular tachycardia. The drug in-
creases CO and decreases MAP, HR, SVR, wedge,
CVP, and PVR. It Paents will almost always receive
a loading dose of 150 mg mixed in D5W given over
10 minutes. The infusion will start at 1 mg/min for 6
hours. If the inial rhythm has resolved, the nurse
should ancipate that the provider will drop the
infusion rate to 0.5 mg/min aer 6 hours, maintain-
ing the drip at 0.5 mg/min for the subsequent 18
hours prior to transioning to oral Amiodarone.
Amiodarone may also be used for paents who ar-
rest with a VF/pulseless VT eology and are unre-
sponsive to debrillaon (American Heart Associa-
on, 2018).
Dilazem
Dilazem is a calcium channel blocker used for
heart rate control in the seng of sinus tachycar-
When giving nitroprusside, it is
important to evaluate the color of the
drug. When nitroprusside turns blue, it
has decomposed into cyanide and
should not be given to the paent.
PRECEPTOR PEARLS

Back to Table of Contents 32
Norepinephrine
(Levophed)
Phenylephrine
(Neo-synephrine)
Epinephrine Dopamine Vasopressin
CO
PAOP
SVR
MAP
HR
➔
CVP
PVR
Receptor
Agonist/ MOA
Primarily alpha,
some beta agonist
properes
alpha Alpha, Beta1,
Beta 2. More
Beta at higher
doses
Alpha, Beta1, Beta2 Vasopressin 1A
Dose/ Titraon 2-20 mcg/min
Titrate by 1-2 mcg/
min
20-200 mcg/min
Titrate by 20 mcg/
min
1-10 mcg/min
Titrate by 1
mcg/min with
provider order
1-20 mcg/kg/min
Titrate by 5 mcg/
kg/min with provid-
er order
0.04 units/min
May see 0.06
units/min; do
not trate with-
out order
Concentraon 8mg/250ml
16mg/250ml
D5W
D5WNS
30mg/250ml
120mg/250ml
D5W
NS
4mg/250ml
8mg/250ml
D5W
NS
400mg/250ml
1600mg/250ml
D5W
NS
60un/100ml
100un/100ml
D5W
Access Central Central Central Central Central
Uses Increase HR and
contraclity to in-
crease systemic
blood pressure
Alpha agonist that
increases arterial
vasoconstricon
Increase heart
rate and con-
traclity.
Alpha, B1,B2
agonist
Posive inotrope
increases contracl-
ity.
B1 agonist (low
dose)
Alpha agonist
(high dose)
V1 agonist that
works on vascu-
lar smooth mus-
cle increase BP
Key Clinical
Points
Nofy team for
levophed doses
greater than 10
mcg/min or for rap-
idly increasing
levophed require-
ments
Can cause bradycar-
dia, not a rst line
drug in post-cardiac
surgery paents
May cause hy-
perglycemia—
trend blood
glucose careful-
ly. May increase
lactate
producon.
Can cause tach-
yarrhythmias.
Consider Use on
high dose
levophed, not to
be used as mono-
therapy.
BOX 3.2 ACTIONS OF SELECT VASOPRESSORS AND INOTROPES

Back to Table of Contents 33
Dobutamine Milrinone Nitroprusside Nitroglycerine
CO
PAOP
SVR
MAP
➔
HR
CVP
PVR
Receptor
Agonist/ MOA
B1 agonist Phosphodiesterase Inhibi-
tor, increases cAMP which
increases Ca++ in heart
Dose/ Titraon 1-30 mcg/kg/min
Titrate with order by 1-2
mcg
0.375-0.75 mcg/kg/min
(can go lower too).
MD order to trate.
0.2-10 mcg/kg/
min
trate for target
BP by 0.1-.0.2
mcg.
25 mcg-400 mcg.
Titrate by 10 to 25
mcg to lower BP
and decrease chest
pain.
Concentraon 250mg/250ml
1000mg/250ml
D5W
NS
40mg/200ml
80mg/200ml
.45%NaCl, NS, D5W
50mg/250ml
100mg/250 ml
D5W
25mg/250ml
100mg/250ml
D5W
NS
Access PIV or Central PIV or central Central Central or PIV
Uses Posive Inotrope, smu-
late B1 adrenergic recep-
tors to increase contracli-
ty and reduce le ventricu-
lar lling pressures in heart
failure, post open heart,
pulmonary congeson
Phospho diasterase-
inhibitor, non-adrenergic .
Increases contraclity,
decrease preload, de-
crease aerload. Use in
low CI, heart failure pa-
ents
Potent-direct vas-
odilator. Increases
CO by decreasing
aerload. Used
inially in the post
-op period to de-
crease BP
In MI paents due
to the reducon in
preload thus reduc-
ing cardiac oxygen
demand. Vasodi-
lates primarily
veins. Reduces BP
and chest pain.
Key Clinical
Points
Can Cause tachy- arrhyth-
mias esp aer 72hrs, en-
sure paent if uid volume
resuscitated.
Monitor for thrombocyto-
penia. When weaning
watch for changes in CI in
3-6 hours
Protect from light,
monitor for low
SaO2 due to
shunng and aci-
dosis. Team aware
if on >1mcg
Can cause head-
aches, interacts
with Viagra. Pre-
scribed some-
mes to help de-
crease spasms
aer an interven-
on.
BOX 3.3 ACTIONS OF SELECT VASODILATORS AND INODILATORS

Back to Table of Contents 34
tachycardia be given IV push over 2 minutes prior to
iniang the drip. Dilazem may be trated be-
tween 5-15mg every 5 minutes unl desired heart
rate response is achieved. Nurses should monitor
the paent for hypotension, parcularly during bolus
administraon.
SEDATION__________________________________
Sedaon infusions are commonly used in crical care
for procedures and to manage anxiety related to
mechanical venlaon. When managing sedaon, it
is important to assess and treat alternave sources
of agitaon such as pain prior to iniang sedaon
therapy (Devlin et al., 2018). Analgesic medicaons
to manage post-operave pain can be found in
Chapter 3. Due to their associaon with ICU deliri-
um, roune use of benzodiazepines should be avoid-
ed. Nurses should assess paent sedaon depth us-
ing the validated Richmond-Agitaon Sedaon Scale
(RASS) at minimum every two hours for paents re-
ceiving connuous sedaon, adjusng the rate of
infusion to meet sedaon targets (Box 3.5). Acons
of select sedaon agents can be found in Box 3.4.
Dexmedetomidine
Dexmedetomidine is a selecve alpha2-agonist used
sedaon. The drug does not have analgesic proper-
es. Paents receiving this drug are arousable to
smulaon and have lower rates of delirium com-
pared to other agents such as benzodiazepines. Due
to the minimal eect on respiratory suppression,
dexmedetomidine is the only sedave agent ap-
proved for use in extubated paents. Dexmedetomi-
dine is started at 0.2 to 0.5 mcg/kg/hour and may be
trated by 0.1 to 0.3 mcg/kg/hr to a maximum dose
of 1.5 mcg/kg/hr. Due to the risk of hypotension and
bradycardia, loading doses and boluses are not rou-
nely administered in the ICU. Nurses should moni-
tor for dose-dependent hypotension and brady-
arrhythmia throughout the duraon of therapy.
Ketamine
Ketamine NMDA receptor antagonist that inhibits
the acon of glutamate, an excitatory neurotrans-
mier. The drug has sedave, analgesic, and amnes-
c properes. Although ketamine may be given
through a variety of routes, it is most commonly
used in the ICU for procedural sedaon or as a con-
nuous infusion for sedaon or analgesia to reduce
opioid use. Ketamine may be connuously infused
at 1-2 mcg/kg/min to maintain desired parameters.
If used for procedural sedaon, a bolus of 1 to 4.5
mg/kg may be ordered. Nurses should monitor for
tachycardia and hypertension with ketamine infu-
sion and report an increased heart rate greater than
110 bpm or an increase in systolic blood pressure
greater than 25 mmHg from baseline. Due to its in-
crease on myocardial oxygen demand, ketamine
should be used with cauon in paents with known
coronary artery disease. Ketamine may also induce
hallucinaons or nightmares in some paents.
SCORE DESCRIPTION
+4
Combave: violent with sta or a threat to
self
+3
Very Agitated: Aempts to remove tubes or
lines; aggressive with sta
+2
Agitated: Frequent, non purposeful move-
ment; ghts venlator
+1
Restless: Visibly anxious or apprehensive
0
Alert and Calm
-1
Drowsy: Not fully alert but sustains eye con-
-2
Light Sedaon: Briey (less than 10 seconds)
-3
Deep sedaon: movement to physical smu-
-4
Unarousable to voice or physical smulaon
BOX 3.5 RICHMOND AGITSTION-SEDATION (RASS) SCALE

Back to Table of Contents 35
Propofol
Propofol is a GABA agonist that has both sedave
and amnesc properes. Propofol may be trated
from an inial dose of 5 mcg/kg/min by 5 to 10
mcg/kg/min every 5-10 minutes unl desired eect
is achieved. The maximum dose of propofol for con-
nuous sedaon in the ICU is 50 mcg/kg/min.
Propofol may cause dose-related bradycardia and
hypotension, parcularly in paents with underly-
ing cardiovascular disease. For this reason, propofol
infusions are never bolused in CVICU paent popu-
laons.
NEUROMUSCULAR BLOCKADE_________________
Neuromuscular blocking agents, or paralycs, are
used in conjuncon with sedaon during rapid se-
quence intubaon (RSI) to facilitate intubaon and
during targeted temperature management to pre-
vent shivering. When used for RSI, nurses may be
asked to give a one-me push of a paralyc agent
under physician supervision. The following agents
are used most commonly in RSI: vecuronium,
rocuronium, and succinylcholine. Of these, suc-
cinylcholine has the most rapid onset, between
30-60 seconds, and the shortest duraon of 6-10
minutes. These characteriscs make it an ideal
choice in RSI. However, the drug may cause a pre-
cipitous rise in potassium and should be avoided
in hyperkalemic paents or those with extensive
ssue trauma. The remaining drugs, rocuronium
and vecuronium, have longer duraons of approxi-
mately 30 minutes, making them appropriate choic-
es for procedures such as bedside trach placement.
Cisatracurium
Cisatracurium is a nondepolarizing paralyc with a
quick onset of 2-3 minutes and a longer duraon of
35-45 minutes. It is the only paralyc rounely giv-
en as a connuous infusion and is part of the tar-
geted temperature management order set to pre-
vent shivering in paents cooled aer cardiac ar-
rest. For paents receiving connuous paralyc,
nurses should monitor the extent of paralysis with a
Train of Four (TOF). Cisatracurium should be iniat-
ed at a rate of 1-2 mcg/kg/minute and trated eve-
ry hour to achieve and maintain a TOF of 1-2
twitches. More informaon regarding TOF manage-
ment can be found in Chapter 5. •
Dosage Onset Peak Duraon Side Eects
Precedex
0.2-0.7 mcg/kg/hr
Max dose 1.5 mcg/
kg/hr
5-10 minutes 15-30 minutes 1-2 hours Bradycardia, hypoten-
sion, oversedaon
Propofol
10-50 mcg/kg/min <40 seconds Unknown 10-15 min Bradycardia, hypoten-
sion, respiratory depres-
sion
Ketamine 1-2 mcg/kg/min 30-40 seconds Unknown 10-15 min Tachycardia, hyperten-
sion, hallucinaons, res-
piratory depression
BOX 3.4 ACTIONS OF SELECT SEDATION AGENTS
A paent should be adequately sedated
prior to giving a paralyc. A Bispectral
Index Range (BIS) monitor is used to
gauge the level of alertness in paralyzed
paents. BIS scores range from 1-100,
with higher numbers correlang with
more alertness. Sedaon should be
trated to achieve a BIS score of 40-60.
PRECEPTOR PEARLS

Back to Table of Contents 36
Use the knowledge gained in this chapter and the following scenario to answer the quesons below. When you
are ready, check you answers on p. 69.
You are caring for a paent who is admied for decompensated heart failure, awaing a heart
transplant. The CCU team places a PA catheter, obtaining the following data: PA pressure 48/32, CVP
12, SVR 2250, CI 1.7. The team starts the paent on milrionone 0.375 mcg/kg/min.
1. The team asks you to shoot another set of numbers one hour aer the drip is iniated. Which set
of numbers represents the ancipated response to the iniaon of milrinone?
A. PA pressure 55/42, CVP 14, SVR 2500, CI 1.5
B. PA pressure 35/24, CVP 12, SVR 1800, CI 2.0
C. PA pressure 45/35, CVP 12, SVR 2400, CI 1.9
2. The following day, the paent receives a heart transplant. The paent is persistently hypotensive in
the OR and arrives to the unit on epinephrine 3 mcg/min and norepinephrine 8 mcg/min. Which of the
following represents the expected hemodynamic response to levophed?
A. Decrease MAP by decreasing SVR
B. Increase MAP by decreasing SVR
C. Increase MAP by increasing SVR
3. Overnight, the team weaned the levophed. This morning the team asks you to turn the epinephrine
down from 3 mcg/min to 2 mcg/min. When shoong your next set of numbers, you expect:
A. The cardiac index to decrease from 2.4 to 2.2
B. The cardiac index to increase from 2.3 to 2.5
C. The cardiac index to remain unchanged
A P P LY Y O U R K N O W L E D G E : CLINICAL CASE STUDY

Back to Table of Contents 37
A R D I A C S U R G E R Y
C
4
Cardiovascular intensive care nurses can expect to
care for paents undergoing a wide variety of cardi-
ac procedures as well as vascular procedures that
require a thoracic approach. This chapter will pro-
vide an introducon to the most common proce-
dures received in the CVICU, highlighng standard
preoperave and immediate postoperave care.
Postoperave complicaons will also be reviewed.
PREOPERATIVE NURSING CARE_________________
Preoperave paent assessment includes a baseline
assessment of heart funcon and comorbidies to
evaluate surgical risk. Standard preoperave labs
include: CBC, CMP, HgA1c, type & screen, and an
urinalysis. Paents taking coumadin at home or pa-
ents receiving heparin preoperavely will also re-
ceive coagulaon studies. Comorbidies such as
COPD, diabetes, renal insuciency, peripheral vas-
cular disease, and heart failure with reduced ejec-
on fracon are associated with increased perioper-
ave risk (Nadim et al., 2018). Addional diagnosc
studies such as 12-lead EKG; echocardiography; CT
or cardiac MRI; cardiac catheterizaon; and pulmo-
nary funcon tests may also be performed pre-
operavely in non-emergent cases. Reviewing pre-
operave results may inform post-operave nursing
assessment, priories, and plan of care.
Paents admied to the ICU prior to surgery require
specic nursing intervenons. Intranasal mupirocin,
used to prevent MRSA infecon, will be ordered eve-
ry 12 hours starng the night before surgery and will
connue unl the paent receives 10 postoperave
doses. Preoperave anbiocs will be ordered to go
to the OR with the paent and will be given by the
anesthesia team within one hour of the rst incision.
Unless the paent has an allergy, standard anbi-
ocs include cefazolin and vancomycin.
Paents should be allowed to shower or receive a
soap and water bath the night before surgery. Aer
the paents have received a soap and water bath,
nurses should provide two preoperave CHG scrubs,
one the night before surgery and one the morning of
surgery as close to the operave me as possible.
Paents should have hair clipped immediately prior
to the second CHG scrub. Paents should be clipped
at minimum four inches wider than any possible inci-
sion. Special aenon should be paid to clipping
groin areas in the event that the surgical team will
need to perform a cut down to the femoral artery.
For urgent or emergent cases, paents should re-
ceive a minimum of one preoperave CHG scrub im-
mediately following paent clipping.
All CVICU paents will be transported to the opera-
ve suite with debrillator pads in place. In lieu of
the standard placement, pads will be placed laterally
on either side of the chest, immediately inferior to
the axilla with the wires tracing up between the
should blades. This placement allows the pads to be
easily accessed during surgery while remaining free
of the surgical eld for a median sternotomy ap-
proach. Paents who will receive mini thoracotomy
approaches should have pads placed in the standard
fashion.
Nurs i ng C a re of t he Pat ie n t U nd e rgo i ng C a rdi a c Surge r y

Back to Table of Contents 38
CORONARY ARTERY BYPASS___________________
Coronary artery bypass (CAB) is indicated for pa-
ents with mulvessel coronary disease or sever sin-
gle vessel disease that cannot be treated with percu-
taneous coronary intervenon (PCI). To perform a
CAB, the surgeon most commonly accesses the tho-
racic cavity via a median sternotomy incision. Vessel
gras, harvested from the le internal mammary
artery, saphenous veins, or radial artery, are used as
conduits to bypass diseased vessels and restore
blood ow to the myocardium. Gras are sewn into
the proximal aorta and routed into the aected cor-
onary artery just distal to the occluded poron of
the vessel.
CAB is usually performed using cardiopulmonary by-
pass, commonly referred to as ‘on pump’ (Figure
4.1). In this procedure, the paent is cooled be-
tween 30-34° Celsius, the heart is stopped using car-
dioplegia, and the aorta is cross clamped. Blood is
drained from the right atrium, oxygenated in the by-
pass machine, and returned to the body via a cannu-
la placed distally to the cross clamped secon of the
aorta. Heparin is used to reduce the risk of clot for-
maon in the circuit. Less frequently, CAB will be
performed without use of the bypass machine, re-
ferred to as an o-pump CAB (OPCAB). In this proce-
dure, the surgeon sews gras into the myocardium
while the heart is sll beang. Although OPCAB may
avoid certain risks associated with cardiopulmonary
bypass, it is associated with higher rates of gra oc-
clusion than tradional CAB (Hardin & Kaplow,
2020). Addionally, a paent may need to be con-
verted to an on-pump procedure if the coronary ar-
teries are determined to be parcularly small, the
le ventricular funcon is poorer than expected, or
uncontrollable arrhythmias develop.
Nursing Consideraons
Anesthesia providers will bring paents to the ICU
immediately aer the chest is closed. The use of car-
diopulmonary bypass simultaneously increases the
risk of bleeding and clot formaon. Bleeding may
occur from inadequate heparin reversal; hemodilu-
on and hemolysis during cardiopulmonary bypass;
and bleeding from anastomosis sites. Hypothermia
Figure 4.1. Cardiopulmonary Bypass. By National Heart Lung and
Blood Institute (NIH)
- National Heart Lung and Blood Institute
(NIH), Public Domain, https://commons.wikimedia.org/w/
index.php?curid=29588210

Back to Table of Contents 39
exacerbates the risk of bleeding due to the inacva-
on of the clong cascade at low temperatures. The
risk for clot formaon is largely due to the acvaon
of platelets and inammatory mediators when blood
interacts with the bypass circuit. Nurses must re-
main vigilant for signs and symptoms of bleeding,
closely monitoring chest tube output (including the
presence of clots), maintaining chest tube patency,
and monitoring paent labs.
Surgical stress and hypothermia increase the risk of
postoperave hyperglycemia, which has also been
shown to increase postoperave morbidity. Insulin
protocol will be iniated on all postoperave pa-
ents to maintain blood sugars within a normal
range and the paent should be rewarmed as quick-
ly as possible. Following cardiac surgery, paents are
at increased risk of renal dysfuncon, hemorrhagic
stroke, and ischemic stroke. It is important, there-
fore, that nurses perform a comprehensive paent
assessment as quickly as possible and at roune in-
tervals. A comprehensive postoperave workow
can be found in Box 4.1. Provider nocaon param-
eters can be found in Box 4.2 on the following page.
VALVE REPLACEMENT_________________________
Valve surgery is performed to either repair or re-
place diseased cardiac valves. Valvular disease is de-
scribed by the aected valve and the funconal mal-
formaon, either stenosis or regurgitaon. Stenosis
describes a valve with anatomic narrowing that im-
pedes forward blood ow within the heart, most
commonly caused by calcicaons on the valve
leaets, congenital abnormalies, or rheumac
heart disease. Regurgitaon describes a valve that
no longer closes appropriately, allowing regurgi-
tant and inecient blood ow through the heart.
Surgical intervenon is indicated when paents
become symptomac or when ventricular ejec-
on becomes severely impeded.
Open valve replacement is typically conducted via
Hook up arterial line to Phillips monitor* Upon Arrival
Hook up EKG, SPO2, CVP & PA to Phillips
monitor*
Hook up chest tubes to sucon, marking ini-
al level
Document inial urine output
Shoot cardiac outputs and print rhythm strip*
Trace IV lines from bag to pump to paent
Check OG tube placement and hook up to
LIWS*
Send CBC, coagulaon studies (if bleeding),
and ABG
Apply Bair hugger if paent <36°
Change NS carrier to D5NS per insulin
Protocol; Slowly decrease rate to 30 ml/hr
Administer 2 gm Magnesium Sulfate per pro-
tocol
Postopera-
ve Phase
Record vital signs, UOP, and CT output q 15
minutes x4 then q 30 minutes unl CT output
< 100 ml/hr
Shoot cardiac outputs Q1 hour x4 then Q4
hour
Trend labs, parcularly electrolytes, and re-
place per protocol as needed
Titrate vasoacve medicaons to SBP & MAP
goals
BOX 4.1 Open Heart Admission Workow
*Items typically delegated to Help All
Keeping ght control of blood pressure
is important post surgery. MAP must be
high enough to perfuse end organs, but
Increased SBP puts increased pressure
on fresh anastomoses., increasing risk of
bleeding.
PRECEPTOR PEARLS

Back to Table of Contents 40
a median sternotomy, however, mitral valve and
aorc valve replacement may be performed through
a thoracotomy incision. Both median sternotomy
and thoracotomy approaches require the use of car-
diopulmonary bypass. Replacement valves are either
mechanical or bioprosthec. Mechanical valves are
more durable but also carry a higher risk of throm-
boembolic events, requiring paents to remain on
ancoagulaon aer surgery. While bioprosthec
valves avoid the need for ancoagulaon, these
valves do not last as long as their mechanical coun-
terparts (Hardin & Kaplow, 2020). The decision to
ulize a bioprosthec or mechanical valve is highly
paent specic and discussed during the pre-
operave paent evaluaon.
In recent years, the advent of transcatheter aorc
valve replacement (TAVR) has revoluonized the
treatment of aorc stenosis in paents whose surgi-
cal risk would otherwise be prohibive to open re-
placement. The success of these procedures has
prompted subsequent clinical trials in lower risk sur-
gical paents, increasing excitement that minimally
invasive valve replacement may be available to more
paents in the near future. TAVR is tradionally per-
formed by accessing the femoral artery and deploy-
ing a specially craed mechanical valve atop the ex-
isng aorc valve. The is conducted in a hybrid OR
with a cardiac surgeon and bypass machine available
if the paent’s clinical condion deteriorates. In-
creasingly, TAVRs are performed under moderate
sedaon and paents are frequently transferred to
cardiac stepdown immediately following sheath re-
moval.
Nursing Consideraons
Open valve replacements carry the same risk prole
of CAB procedures and paents should be moni-
tored accordingly. An open heart admission work-
ow can be found in Box 4.1 on the previous page
and provider nocaon parameters can be found in
Box 4.2. In addion to the standard risks of cardio-
pulmonary bypass, paents undergoing valve re-
placement are at increased risk of thromboembolic
events, infecve endocardis, paravalvular leak, atri-
al brillaon, and AV block post procedure (Gaasch
& Zoghbi, 2017). To migate the risk of brady-
arrhythmia postoperavely, pacing wires are usually
kept in place for at least 48 hours aer surgery. Im-
portantly, the acute hemodynamic changes incurred
from replacing a chronically dysfunconal valve may
prompt hemodynamic instability in the immediate
postoperave phase. This requires careful volume
management; close hemodynamic monitoring; and
either inotropic or vasodilatory support (Hardin &
Kaplow, 2020). In spite of these risks, paents are
usually extubated within 6 hours of arrival to the
unit and discharged to stepdown POD 1 or POD 2.
Signs & Symptoms of CVA/TIA CI<2.0 or significant decrease
SVO2<60 SBP<90 or >150
HR >100 or <55 (w/rhythm strips) Loss/Change of extremity pulse, temp, movement
Starting vasoactive drips not initiated prior to arrival in
CVICU
Levo>10 mcg/min or significant increase
Nipride >2 mcg/kg/min Propofol >50 mcg/kg/min
Chest Tube output >100cc/hr or >25cc/15 min UOP<30 cc/hr
Oxygen Requirement >6LNC after extubation Sat<92%
FiO2 >60% while ventilated pH<7.25
pCO2 >55 pO2<60
PCV <25 or Hgb <7
K<3 or >5.5
BOX 4.2 Provider Nocaon Parameters

Back to Table of Contents 41
TAVR carries a relavely high risk of stroke and post-
operave dysrhythmias. Neurological checks should
be every hour in the immediate postoperave phase
and rounely thereaer. Any neurological changes
should be reported immediately to the provider
team and a STAT CT should be ordered. Addionally,
nurses should carefully monitor TAVR access sites
for signs of bleeding, including signs of retroperito-
neal bleeding, due to the large sheath sizes ulized
for the procedure.
HEART TRANSPLANTATION____________________
Heart transplantaon is indicated for paents who
have failed medical treatment of their heart failure
and connue to exhibit signicant symptoms and
reducon in quality of life, dened as New York
Heart Failure Associaon (NYHA) class III or IV. Fur-
ther informaon about heart failure classicaon
and medical management of heart failure can be
found in Chapter 5. Heart transplantaon may also
be indicated for paent who exhibit recurrent ar-
rhythmias refractory to treatment, cardiomyopathy,
or congenital heart disease in which tradional re-
pair techniques are not applicable (Hardin & Kaplow,
2020). Paents undergo extensive tesng to deter-
mine their eligibility for heart transplantaon, in-
cluding but not limited to: evaluaon of comorbidi-
es such as pulmonary hypertension, diabetes, and
renal dysfuncon; immunizaon status; psychologi-
cal evaluaon as well as an evaluaon of social and
nancial support. Once approved, a transplant coor-
dinator will work with the Organ Procurement and
Transplantaon Network (OPTN) to place the paent
on the naonal waitlist. Paents are listed according
to the severity of their illness, ranked as status 1-6
by the OPTN. Status 1 paents represent the most
acutely ill paents, such as paents who are ad-
mied to the ICU for non-dischargeable bi-
ventricular support. A complete list of organ alloca-
on criteria for medical urgency can be found in Box
4.3 on the following page. Organs are allocated by
Figure 4.2. A) Pictorial diagram showing where the diseased heart is removed for transplant. B) Anastomoses of the
transplanted heart after implantation. By National Heart Lung and Blood Institute (NIH) - National Heart Lung and Blood
Institute (NIH), Public Domain, https://commons.wikimedia.org/w/index.php?curid=29588222

Back to Table of Contents 42
geographic region, paent size, anbody matching,
and paent acuity. Once an organ has been
matched, a member of the surgical team will travel
to the donor to conduct a nal evaluaon of the or-
gan for transplantaon and the transplant coordina-
tor will contact the paent to alert them that an or-
gan has become available.
Nursing Consideraons
Heart transplantaon is a 6-8 hour operaon. Pa-
ents will arrive to the ICU prior to transplant for
preoperave care. In addion to the usual preopera-
ve nursing care, ICU nurses can expect to send ad-
dional paent-specic anbody labs and assist with
line inseron prior to the paent going to the OR. If
the paent is admied with a PICC line for IV inotro-
py, this line should be removed prior to the paent
going to the OR suite.
The postoperave course of heart transplants pa-
ents is variable and dependent upon recipient, do-
nor, and operave factors. Recipients with preopera-
ve pulmonary hypertension, renal dysfuncon, or
mechanical circulatory support (MCS) are more likely
to have postoperave complicaons. Older donor
age, longer ischemic mes, and recipient-donor mis-
match may also inuence postoperave outcomes
(Hardin & Kaplow, 2020). Bleeding, hypovolemia,
and right ventricular dysfuncon are common com-
plicaons following heart transplantaon. To protect
the right ventricle, paents may arrive to the ICU on
inhaled vasodilators such as Flolan or nitric oxide.
Volume status must also be delicately managed in
the post-heart transplant paent. Hypovolemia may
decrease coronary artery perfusion to a stunned my-
ocardium, however, hypervolemia may overwhelm a
stunned right ventricle (Hardin & Kaplow, 2020).
Lastly, nurses can expect that the post-heart trans-
plant paent will arrive to the ICU with epicardial
pacing between 100-110 bpm. Pacing at a higher
rate not only augments cardiac output postopera-
vely but also smulates the return of sinoatrial
node funcon aer the vagus nerve has been sev-
ered during transplant.
In addion to standard monitoring of the post-
cardiotomy paent, ICU nurses must closely moni-
tor for signs of rejecon. All paents will receive im-
munosuppressive agents immediately prior to trans-
plant (inducon) and for the duraon of their life
thereaer. An-rejecon agents of choice include
calcineuron inhibitors (CNI), anproliferave agents,
and steroids. A list of select an-rejecon agents,
including administraon and specialized considera-
ons, can be found in Box 4.4. Hyperacute rejecon,
caused by allogra mismatch, is rare and occurs
within minutes to hours of transplantaon. Acute
cellular rejecon is more common and occurs most
frequently within the rst 6 months of transplant
(Eisen, 2017). For this reason, cardiac biopsies are
BOX 4.3 OPTN Adult Heart Allocaon Criteria
Status 1 VA ECMO
Non-dischargeable biventricular support
MCS with life-threatening arrhythmia
Status 2 Non-dischargable LVAD
IABP or other percutaneous MCS
Life-threatening arrhythmias w/o MCS
TAH, BiVAD, or RVAD
Status 3 Mulple inotropes or single inotrope with connu-
ous hemodynamic monitoring
VA ECMO aer 7 days; Non-dischargeable LVAD
aer 14 days
MCS device with clinical complicaon
Status 4 Dischargeable LVAD
Inotropes without hemodynamic monitoring
Re-transplant, congenital heart disease, amyloido-
sis, certain cardiomyopathies
Status 5 Those waitlisted for mulple organs at a single
Status 6 All remaining candidates

Back to Table of Contents 43
obtained at roune intervals for the rst 6 months
aer transplant. Anbody-mediated rejecon is less
common, but may occur at any me aer transplan-
taon, necessitang lifelong immunosuppression. It
is important to note that mild rejecon is oen
asymptomac and only discovered by roune sur-
veillance. Symptomac or more severe rejecon may
present with new or worsening heart failure symp-
toms from primary gra dysfuncon.
Transplant recipients receive extensive educaon
preoperavely. Nonetheless, ICU nurses must be
prepared to reinforce this educaon during the im-
mediate postoperave course. Since transplant pa-
ents are immunosuppressed, nursing sta, paents,
and family members must pracce meculous infec-
on prevenon measures. In addion to an-
rejecon medicaon, paents will receive prophylac-
c anbiocs and anviral medicaon immediately
following transplant.. Paents will also need to avoid
undercooked meats and wash all fruits and vegeta-
bles prior to consuming them. Raw foods should be
avoided immediately aer transplant. Finally, cardiac
rehab is an important part of postoperave recovery.
All Vanderbilt transplant paents stay locally aer
discharge for frequent appointments with the outpa-
ent heart transplant team and to aend cardiac re-
hab at the Dayani Center.
Medicaon Administraon Special Consideraons Class
Cyclosporine PO or IV
Time sensive: Q12 hours,
dosed at 0600 and
1800
Draw daily lab troughs at 5 am
Use gloves when handling medicaon; do not crush;
must order suspension for per tube
CNI
Tacrolimus PO or Sublingual
Time sensive: Q12
hours, dosed at
0600 and 1800
Draw daily lab troughs at 5 am
Use gloves when handling; do not crush, must open
capsule and deliver contents sublingual if una-
ble to swallow pill (wear globes & N95 to do so).
CNI
Mycophenolate PO or IV (slowly over 2
hours)
Time sensive: Q12
hours, dosed at
0600 and 1800
Use care when discarding. Put IV tubing and bag in
yellow containers.
Use gloves when handling; do not crush
An-
proliferave
Methylprednisolone
PO or IV (may be given
IVP or piggback de-
pending on dose
Ordered daily
Closely monitor blood glucose
Dose weaned by team based on cardiac biopsies
May be increased if s/s of rejecon
Steroid
THYMOglobulin
IV (1
st
dose over 6 hrs,
remaining doses
over 4-6 hrs.)
Used for inducon or steroid-resistant rejecon
MUST use Central access and 0.22 micron lter
Premedicate with Tylenol, Benadryl, steroids. Ana-
phylaxis kit at bedside.
Polyclonal
Anbody
Basiliximab
(Simulect)
IV infusion over 30
minutes
Ordered as inducon ONLY
No premeds needed
Monoclonal
Anbody
BOX 4.4 Select Immunosuppressive Agents

Back to Table of Contents 44
LUNG TRANSPLANTATION_____________________
Lung transplantaon is indicated for paents who
have end –stage lung disease that is no longer re-
sponsive to medical management.. This may include
paents with COPD, cysc brosis, primary pulmo-
nary hypertension, or sarcoidosis. The pre-transplant
evaluaon for lung transplantaon is similarly rigor-
ous to that for heart transplantaon. As with heart
transplantaon, paents are listed according to the
acuity of their clinical status and matched according
to geographical region, organ size, and anbody
screening. Upon arrival to the unit, paents should
receive roune pre-operave care, including the ad-
dional pre-transplant labs. The lung transplant
team prefers their paents to be clipped in the OR
immediately prior to the rst incision.
Nursing Consideraons
Lung transplants are performed using a clamshell
incision, which is parcularly painful postoperavely.
For this reason, all lung transplant paents receive
an epidural prior to extubaon. The Vanderbilt Pain
Management service will insert and set up the epi-
dural in the ICU. Nursing sta are responsible for
monitoring the epidural site at roune frequency,
documenng epidural infusion volumes, and chang-
ing epidural medicaon bags. An epidural quick
guide can be found in Box 4.5.
Much of the postoperave care of lung transplant
paents is geared toward avoiding reperfusion injury
to the allogra. Reperfusion injury presents as se-
vere pulmonary edema of non-cardiac eology with
reduced lung compliance and impaired gas ex-
change. To this end, paents will arrive to the unit
on pulmonary vasodilators and will likely be extubat-
ed with these agents in place. Lung protecve ven-
laon strategies and early extubaon also prevent
unnecessary barotrauma to the new organ. Con-
servave uid resuscitaon will also prevent reper-
fusion injury and nurses should expect to keep lung
paents drier than other populaons, somemes at
the expense of increased pressor ulizaon in the
short term. Paents should not be wedged to avoid
unnecessary pressure on the anastomosis site at the
pulmonary artery.
Aer extubaon, paents will be kept strictly NPO
unl a swallow evaluaon can be completed to de-
crease the risk of aspiraon. As with other trans-
plants, meculous infecon prevenon strategies
To Unlock the Key-
pad:
-Press STOP
-Enter Vanderbilt Universal Code
To View the
Current Program
-Press OPTIONS
-Press 1 for REVIEW PROGRAM
To Change the
Program:
-The pump must be stopped and
the keypad unlocked before the
program can be changed
-Press CHANGE
-Press #3 for CHANGE PROGRAM
-Press ENTER at each screen for
variables you want to change
-Use the down arrow to review
the revised program
-Press ENTER
To Change the
Empty
Container:
-The pump must be stopped and
the keypad unlocked
-Press CHANGE
-Press #1 for NEW CONTAINER
-Press START
-The pump will resume the previ-
ously programmed delivery at
the previously selected container
size
To Review
History/Event Log:
-Press OPTIONS
-Press #2 HISTORIES
-Press #1 HISTORY, scroll through
the event log by pressing the
down arrow
BOX 4.5 Epidural Quick Guide

Back to Table of Contents 45
should be employed. Paents will receive a similar
immunosuppression regimen to that previously de-
scribed in this chapter and should be educated re-
garding their medicaons. Lung transplant paents
will also need to follow a transplant-approved diet,
including fully cooked meats and stringent washing
of fresh fruits and vegetables.
VASCULAR SURGERY__________________________
A number of vascular diseases, most commonly aor-
c aneurysm and dissecons, are also cared for in
the CVICU. An aorc aneurysm is dened as a dila-
on of the aorta at least 50% of its normal size
(Sidebotham, Mckee, Gillham, & Levy, 2007). Aneu-
rysms are described by their anatomical locaon.
Ascending aorc and aorc arch aneurysms arise
between the aorc valve and the le subclavian ar-
tery. Descending thoracic and thoracoabdominal an-
eurisms occur distal to the le subclavian artery.
By contrast, an aorc dissecon is caused by a tear
in the inmal layer of the aorc wall that develops a
false lumen. As with aneurysms, dissecons are clas-
sied by anatomical locaon. Type A dissecons
aect the ascending aorta and Type B dissecons
aect the descending aorta. Both diseases are mul-
factorial in origin. Paents may have genec pre-
disposion to the disease, congenital malformaon,
or family history of vascular disease. Comorbidies
such as obstrucve lung disease and a number of
modiable risk factors such as hypertension and
smoking also impact disease prevalence. In the early
stages, aorc malformaons involving the descend-
ing thoracic and abdominal aorta may be treated
medically by managing hypertension and monitoring
the malformaon with roune CT scanning. Malfor-
maons involving the ascending aorta or large mal-
formaons in any part of the descending aorta re-
quire surgical repair.
Nursing Consideraons
Paents receiving open thoracic and thoracoab-
dominal repair will experience signicant uid shis
and potenal blood loss, requiring generous volume
resuscitaon. Neurovascular checks should be per-
formed every hour during the immediate postopera-
ve phase due to the high risk of ischemia following
aorc cross clamp. Up to 40% of paents undergoing
open repair will experience acute kidney injury
(Becker, 2016). Nurses should closely monitor in-
take , output, BUN, and creanine in these paents.
Paents will remain strictly NPO unl they are pass-
ing gas. Nurses should educate paents regarding
pulmonary toilet and facilitate ambulaon TID begin-
ning POD 2.
Thoracic endovascular aorc repair (TEVAR) involves
the inseron of a vascular gra into the aected re-
gion of the aorta via the femoral artery. Endovascu-
lar repair demonstrates decreased surgical risk
across all categories, decreased risk of blood loss,
and is an opon for many descending aorc malfor-
maons. Paents will arrive to the ICU extubated
and transfer to the oor POD 1. As with open repair,
nurses should conduct frequent neurovascular
checks postoperavely.
All thoracic aorc repairs, including TEVAR, will ar-
rive to the unit with a lumbar drain in place due to
the risk of spinal chord ischemia during aorc cross
clamp or gra placement. The lumbar drain will re-
main in place for 24 hours aer the procedure. Nurs-
es should monitor output and CSF pressure hourly
while the drain is open, reporng any output greater
than 50 ml in 4 hours to the vascular team. Unless
complicaons arise, the vascular team will clamp the
lumbar drain POD 1. Nurses should connue neuro-
vascular checks at roune frequency while the drain
is clamped and monitor CSF pressure connuously
unl the drain is removed. If any neurovascular
changes are noted, the nurse should increase the
frequency of the neurovascular checks to Q 15
minutes and nofy the vascular team immediately.•

Back to Table of Contents 46
Use the knowledge gained in this chapter and the following scenario to answer the quesons below. When you
are ready, check you answers on p. 69.
You land a paent from the OR following coronary artery bypass and mitral valve replacement. Your
Help All places the paent on the monitor, applies sucon to your chest tubes and OG tube, and
sends a loaded ABG and CBC to the lab.
1. You expect to perform all of the following nursing intervenons within the rst hour EXCEPT:
A. Assess chest tube output every 15 minutes and report output greater than 100 ml/hr
B. Extubate the paent if the STAT ABG is within normal limits
C. Shoot cardiac indices every hour and nofy the provider if the index is less than 2
2. As you prepare to assess your paent, you reect that paents receiving valve replacement surgery
are at parcularly high risk for:
A. Acute Kidney Injury
B. Hyperglycemia
C. Ischemic Stroke
3. Your paent’s ABG results with the following values: pH 7.36, PO2 96%; PaCO2 42; HCO3-24; K 2.9
PCV 28. Based on these values, you ancipate:
A. Nofying the provider and using the venlator protocol to increase the respiratory rate
B. Nofying the provider and using the nursing algorithm to administer a unit of blood
C. Nofying the provider and using the electrolyte replacement protocol to replete potatassium
A P P LY Y O U R K N O W L E D G E : CLINICAL CASE STUDY

Back to Table of Contents 47
E D I C A L C A R D I O L O G Y
M
5
Nurs i ng C a re of t he M ed i ca l Ca rd iol o g y Patie nt
Vanderbilt University Hospital CVICU cares for both
cardiovascular surgery and medical cardiology ser-
vice lines. This chapter will provide an introducon
to common paent diagnoses cared for in the medi-
cal cardiology service line, including paents ad-
mied for acute coronary syndrome, cardiogenic
shock, and post-arrest paents. Current pracces in
heart failure management and cardiomyopathy will
also be reviewed.
ACUTE CORONARY SYNDROME________________
Acute coronary syndrome (ACS) is an umbrella term
that covers a wide variety of clinical diagnoses,
ranging from unstable angina to acute myocardial
infarcon. Paents with acute coronary syndromes
present with signs and symptoms of myocardial is-
chemia such as chest pain; pain down one or both
arms; shortness of breath; fague; nausea; vom-
ing; or anxiety. Women are more likely to present
with atypical symptoms such as epigastric, back, or
jaw pain. Notably, those with diabetes, postopera-
ve paent, and the elderly may not present with
typical signs and symptoms of ischemia and should
be treated with a lower threshold for addional
tesng. Paents presenng with these symp-
toms should receive a STAT EKG and cardiac
markers upon arrival to the facility or upon
presentaon of symptoms if already admied.
Unstable Angina
Stable angina is dened by chest pain that fol-
lows a predictable paern such as pain during
exeron or mes of stress. Stable angina may be
relieved by rest or nitroglycerin. By contrast, unsta-
ble angina is characterized by chest pain at rest or
unpredictable chest pain that may or may not be
relieved with nitroglycerin. Paents with unstable
angina may present with new or worsening chest
pain symptoms, however upon further invesga-
on, do not demonstrate increased troponin. Pa-
ents with unstable angina may or may not demon-
strate transient EKG changes. Once the diagnosis of
unstable angina has been determined, management
focuses on resolving the acute ischemic pain and
assessing whether the paent demonstrates acute
hemodynamic compromise. Supplemental oxygen
should be supplied to paents with oxygen satura-
on less than 94%. Unless contraindicated, paents
will receive a beta blocker within 24 hours to de-
crease myocardial oxygen consumpon. Paents
unresponsive to sublingual nitroglycerin or paents
Troponin, indicave of myocardial injury,
may not rise for 2-3 hours aer the
onset of myocardial infarcon. A paent
who presents soon aer onset of chest
pain may not yet demonstrate a
troponin rise and should be monitored
closely.
PRECEPTOR PEARLS

Back to Table of Contents 48
who are persistently hypertensive will receive a ni-
troglycerin drip. Following resoluon of the acute
incident, paents will remain in observaon for at
least 12 hours to trend cardiac markers and monitor
for symptom recurrence.
Non-ST Elevaon MI
Non-ST Elevaon MI (NSTEMI) is characterized by
new or worsening, unrelenng chest pain with posi-
ve biomarkers and either ST depression or T wave
inversion on EKG. NSTEMI is most commonly caused
by a non-occlusive plaque rupture that signicantly
alters blood ow to a poron of the myocardium.
From a nursing standpoint, the care of the NSTEMI
paent is much the same as the care of a paent
with unstable angina. Paents who demonstrate he-
modynamic compromise will likely undergo emer-
gent coronary angiography for potenal percutane-
ous coronary intervenon (PCI). Paents who are
hemodynamically stable will be admied to an inpa-
ent unit for medical management and undergo cor-
onary angiography within 24 hours.
ST-Elevated MI
ST elevated MI is characterized by unrelenng chest
pain with posive biomarkers and elevaon of ST
segment in two or more conguous leads in a 12-
lead EKG. An ST-elevated infarcon, commonly
known as a STEMI, is indicave of complete loss of
blood ow distal to a ruptured coronary plaque. If
blood ow is not restored, the poron of the myo-
cardium supplied by the aected coronary artery will
die. For this reason, the team will acvate a STEMI
alert and the cardiac cath lab immediately if a STEMI
is suspected. The naonal standard for reperfusion,
known as door-to-balloon me is 90 minutes. De-
pending on the aected vessel and the me to
reperfusion, STEMI paents are more likely to be-
come unstable than other ACS paents. For this rea-
son, all STEMI paents post PCI will be admied to
the CVICU for close paent monitoring following in-
tervenon.
Care of the Post-Intervenon Paent
When a diagnosc catheterizaon is warranted, the
Standard Raonale
Acute Myocardial Infarcon (AMI)
Aspirin within 24 hours of arrival
Decreases clong by inhibing platelet aggregaon, decreas-
es vasoconstricon, and decreases the risk of death during
AMI by 70%
Beta-Blocker within 24 hours of arrival
Given early (IV) in the course of AMI, decreases size of infarct,
decreases the incidence of arrhythmias, and decreases the
risk of cardiac rupture
PCI for ST elevaon within 90 minutes of arrival Early reperfusion decreases the size of the infarct
Aspirin prescribed at discharge Decreases clong by inhibing platelet aggregaon
Beta-Blocker prescribed at discharge
Decreases myocardial oxygen demand by decreasing heart
rate and contraclity, protects against sudden death by de-
creasing electrical impulse conducon, and decreases the risk
of a recurrent MI
ACE Inhibitor/Angiotensin Receptor Blocker (ARB)
prescribed for LV dysfuncon (EF < 40%)
Preserves LV funcon by decreasing remodeling (formaon of
scar ssue)
Smoking cessaon counseling provided for current
smokers and those that have quit within 12 months
of admission
Risk factor modicaon – nicone promotes clong, vasocon-
stricon, and increased myocardial oxygen demand
BOX 5.1 ACS Standards and Raonales

Back to Table of Contents 49
intervenonal cardiologist and the cardiac catheteri-
zaon team will choose either a femoral or radial
approach based on the paent’s anatomy, catheteri-
zaon urgency, and ancipated intervenon. Radial
artery catheterizaon has a lower risk prole post-
catheterizaon and allows the paent to ambulate
faster upon arrival to the unit.
Radial catheterizaons will have a TR band (Figure
5.1) applied to the sheath exit site prior to arrival to
the unit to maintain hemostasis. Upon arrival to the
unit, the cardiac cath team will report the me that
the TR band was applied as well as the me that the
TR band may be deated. At the appropriate me,
the nurse should deate air from the TR band at reg-
ular intervals unl air is completely removed from
the device. During this phase, nurses should monitor
for signs of bleeding and re-inate air as needed to
maintain hemostasis. For more detailed informaon
regarding TR band management, nurses should refer
to the associated order set and TR band policy, avail-
able in PolicyTech.
Intervenonal cardiologists may ulize a variety clo-
sure devices following femoral artery sheath remov-
al. Angioseal, an absorbable anchor and collagen
plug, is the most common closure device ulized.
Nurses may also see other brands of collagen plugs
such as Mynx or Starclose. All collagen plus dissolve
within 30-60 days following deployment, once the
vessel has healed. For larger sheath sites, cardiolo-
gists may choose to use a suture-style closure device
such as Perclose. Regardless of closure device, all
paents following femoral sheath removal should
remain at for a minimum of two hours following
diagnosc angiography and 6 hours following PCI.
During this me, nurses should closely monitor for
signs of oozing or hematoma formaon. If bleeding
or hematoma is noced, nurses should apply pres-
sure 1-2 cm above the puncture site and nofy the
provider team immediately. Less commonly, femoral
sheath removal may result in a retroperitoneal
bleed, characterized by ank pain and hypotension
unresponsive to uid bolus. If a retroperitoneal
bleed is suspected, nofy the provider team immedi-
ately.
In addion to monitoring sheath removal sites,
CVICU nurses can expect to administer and provide
educaon about standard post-intervenon medica-
ons. A complete list of ACS standards and their ra-
onales can be found in Table 5.1. Paents who
have received contrast in the lab will receive a bolus
of 1 Liter of crystalloid following intervenon to pre-
vent contrast-induced nephropathy.
TARGETED TEMPERATURE MANAGEMENT________
Targeted temperature management (TTM) has been
invesgated experimentally and used clinically for
over 100 years to treat paents who do not respond
neurologically following cardiopulmonary arrest. The
raonale for the clinical applicaon of TTM is to de-
crease a paent’s metabolic rate following arrest,
allowing the injured brain to heal. For each degree a
paent is cooled, there is a 6% reducon in end or-
gan oxygen consumpon. The Vanderbilt Targeted
Temperature Management protocol is based on the
2010 guidelines and protocols established by the
American Academy of Neurology. The following sec-
on of this manual includes a high-level overview of
the protocol, highlighng clinically salient points for
Figure 5.1 TR Band.

Back to Table of Contents 50
implementaon. It is strongly recommended that a
copy of the complete protocol be printed for refer-
ence prior to iniaon of TTM. A complete copy of
the TTM protocol can be found on the CVICU web-
site.
Iniaon and Cooling
Based on a large body of evidence, VUMC has in-
cluded specic inclusion and exclusion criteria to
iniate TTM. A complete list of inclusion and exclu-
sion criteria can be found in Box 5.2. Those paents
who qualify for protocol iniaon will be cooled
with the Arc Sun Targeted Temperature Manage-
ment System. Paents will be cooled to 33 degrees
for 24 hours from their inial downme.
During the cooling phase, paents will experience a
number of physiologic changes. As paents cool to
33 degrees, they oen become bradycardic. This
may or may not result in hemodynamic compromise
for the paent. In this populaon, hemodynamic
instability is dened as hypotension requiring nore-
pinephrine greater than 15 mcg/min or hypotension
that requires the addion of a second inotrope or
vasopressor. If hemodynamic instability presents in
these paents, the protocol prompts providers to
consider increasing the targeted temperature from
33 degrees to 36 degrees.
All TTM paents are paralyzed and sedated through-
out the cooling phase of treatment. This is, in part,
to prevent shivering that increases the paent’s
metabolic rate while the paent is hypothermic. All
paents have connuous BIS monitoring to measure
level of sedaon, trang sedaon to achieve a BIS
of 40-60. Train of Four (TOF), a peripheral nerve
smulator, is ulized to monitor the degree neuro-
muscular blockade throughout the duraon of the
cooling phase. The TOF may be applied to the ulnar
nerve (Figure 5.2) to elicit a thumb twitch or the fa-
cial nerve to elicit an eyelid twitch (Figure 5.3). TOF
is monitored every hour while a paent is receiving
paralycs, trang paralycs to achieve one to two
twitches for every four smuli given. The number of
MA’s required to elicit a response should be noted
in the paent’s chart. Paralycs and sedaon should
never be disconnued while a paent is cold.
Lastly, it is important to recognize that hypothermia
causes electrolytes to shi across the cellular mem-
brane, requiring judicious use of electrolyte replace-
ment protocols. Potassium is not replaced unless
the serum level falls below 2.8 mEq/L and should be
checked every 6 hours for the rst 24 hours.
Rewarming
The paent will be rewarmed at 0.25 degrees per
hour 24 hours aer the me of their arrest. During
the rewarming phase of treatment, paents are like-
ly to experience rebound hyperthermia. For this rea-
Inclusion Criteria Exclusion Criteria
Arrest with primary cardiac eology Arrest from non-cardiac eology
Ability to iniate TTM within 6-12 hours of ROSC
Paents with known terminal illness, bleeding issues, recent
head trauma or a traumac arrest
18 years or older Pregnancy
Unresponsive and not following commands aer
ROSC
Awakens spontaneously with purposeful movement and abil-
ity to follow commands
Esmated me from arrest to ROSC <60 minutes
Unwitnessed arrest with suspected prolonged downme AND
inial rhythm unshockable
Inial temperature less than 34C
BOX 5.2 Inclusion and Exclusion Criteria for TTM

Back to Table of Contents 51
-son, the Arc Sun pads are kept on the paent for
up to 72 hours aer rewarming and acetaminophen
Is scheduled to control for fever. Paralyc infusion
will be disconnued when the paent reaches 36
degrees and sedaon will be weaned when the TOF
returns to four twitches. During any phase of treat-
ment, shivering or suspicion of shivering may be
treated by counter warming extremies with the
Bair Hugger or with demerol.
In the event that a paent needs to be rewarmed
outside of the protocol, the Withdrawal Guidelines
When Disconnuaon Determinaons Are Made
Aer Therapy Iniaon algorithm can be found at
the back of the protocol. Neuroprognocaon is
withheld unl 72 hours aer rewarming to allow
adequate me for paralycs and sedaon to be me-
tabolized.
Management of the Heart Failure Paent________
Heart failure is dened as the inability of the heart
to provide adequate cardiac output to meet the
metabolic demands of end organs. Heart failure is
classied anatomically, physiologically, and by pa-
ent symptomology.
Heart Failure Classicaons
Anatomically, heart failure is categorized as either
le ventricular failure, right ventricular failure, or bi-
ventricular failure. Paents with le ventricular fail-
ure classically present with pulmonary symptoms
such as dyspnea on exeron, orthopnea, or pulmo-
nary edema. Conversely, paents with right ventric-
ular failure classically present with systemic symp-
toms such as ascites or peripheral edema. Paents
with bi-ventricular failure will present with symp-
toms from both categories.
Physiologically, heart failure is categorized as systol-
ic or diastolic hear failure based on the aected por-
on of the cardiac cycle. Systolic hear failure repre-
sents a problem with the heart’s ability to pump and
Figure 5.2 Placement of TOF electrodes along the ulnar
nerve. From Wiegand, D.L. [Ed.]. [2017]. AACN procedure manual for
high acuity, progressive, and critical care [7th ed.]. St. Louis: Elsevier.
Figure 5.3 Placement of TOF electrodes along the facial
nerve. From Wiegand, D.L. [Ed.]. [2017]. AACN procedure manual for
high acuity, progressive, and critical care [7th ed.]. St. Louis: Elsevier.

Back to Table of Contents 52
may be caused by a variety of factors including but
not limited to ischemic heart disease, valvular dis-
ease, or poorly controlled hypertension. Systolic
heart failure is also referred to as heart failure with
reduced ejecon fracon (HFrEF) and is character-
ized by an ejecon fracon (EF) less than or equal to
40%.. By contrast, diastolic heart failure represents a
problem with the heart’s ability to ll. Diastolic heart
failure may be caused by a variety of factors, includ-
ing but not limited to restricve cardiomyopathies or
valvular diseases. Diastolic heart failure is also re-
ferred to as heart failure with preserved ejecon
fracon (HFpEF) and is characterized by an EF great-
er than or equal to 50%.
Lastly, heart failure is described by the extent to
which it aects the paent’s funconality. One com-
mon scale used to quanfy heart failure symptoms is
the New York Heart Failure Associaon (NYHA) scale.
This scale ranks paent symptomology from Class I
(least severe) to Class IV (most severe). The Ameri-
can Heart Associaon priorizes certain diagnosc
tests and treatments based partly on a paent’s NY-
HA scale. Providers may reference these recommen-
daons in addion to the paent’s complete clinical
picture to escalate a treatment plan for a paent
admied with a diagnosis of heart failure.
Diagnosc Criteria
A diagnosis of heart failure is made based on com-
prehensive clinical exam and diagnosc evaluaon.
Serial weights and jugular vein distenon may be
used to establish and trend volume status in addi-
on to a paent’s subjecve symptoms. A variety of
tests and procedures may be ordered to evaluate
heart funcon. Biomarkers such as B-type natriurec
pepde (BNP) as well as other natriurec pepdes
have diagnosc value to establish the inial pres-
ence and degree of heart failure. In the acute
seng, BNP will be drawn at minimum on admission
and prior to discharge. Recent data also suggests
that BNP may have value as a primary screening
mechanism for heart failure (Yancy et al., 2017). Ele-
vated troponin levels may indicate cardiac strain
causing ischemia or necrosis (Yancy et al., 2017). In
addion to biomarker tesng, transthoracic echocar-
diography and chest x-ray as well as le and right
heart catheterizaons have diagnosc and prognos-
c value.
Treang Heart Failure
ACE inhibitors and beta blockers remain a mainstay
of heart failure management and have been shown
to decrease both morbidity and mortality in this vul-
nerable paent populaon (Yancy et. al., 2013).
ACE inhibitors work to counteract the acvaon of
the renin-angiotensin-aldosterone system (RAAS)
triggered by pathophysiologic changes in heart fail-
ure. Beta blockers slow heart rate and decrease
blood pressure, reducing strain on the heart and in-
creasing lling me in paents with reduced ejecon
fracon. Paents who cannot tolerate ACE inhibitors
may alternavely be prescribed Angiotensin Recep-
tor Blockers (ARB) or a combinaon of hydralazine
and isosorbide dinitrate.
As heart failure progresses, the addion of aldoste-
rone antagonists and diurecs may be used to fur-
ther counteract uid retenon in heart failure. ICD
placement is recommended for paents with non-
ischemic dilated cardiomyopathy and paents post
MI with le bundle branch paern and EF less than
35% to reduce the risk of sudden cardiac death
(Yancy et. al., 2013). Paents with heart failure re-
fractory to typical medical treatments may be candi-
dates for IV inotropy, mechanical circulatory sup-
port, or heart transplantaon.
CARIODMYOPATHY__________________________
Cardiomyopathy is a disease process in which the
structure of the heart is changed in the absence of
ischemic disease, congenital disease, or hypertensive
remodeling (Cooper, Mckenna, and Yeon, 2019).

Back to Table of Contents 53
Cardiomyopathy is categorized as hypertrophic, di-
lated or restricted. Dilated cardiomyopathy demon-
strates marked dilaon, decreased wall thickness,
and impaired contraclity of the myocardium. By
contrast, hypertrophic cardiomyopathy demon-
strates thickening of the le ventricular wall that
impedes diastolic lling. Hypertrophic cardiomyopa-
thy may assume an obstrucve morphology known
as HOCM in which the ventricular septum obstructs
the aorc oulow tract with contracon. Lastly, re-
stricve cardiomyopathy is characterized by im-
paired ventricular lling in the absence of hypertro-
phy or dilaon. The treatment of cardiomyopathy
addresses the type of dysfuncon created and, if
known, treat the root cause of the myopathy.
CARDIOGENIC SHOCK_________________________
Cardiogenic shock is a shock condion in which car-
diac output is acutely unable to meet the oxygen
demands of the end organs. Cardiogenic shock is
characterized by high preload (CVP) as uid congests
the heart. As a compensatory mechanism, the pe-
ripheral vasculature constricts in response to low
cardiac output, increasing SVR. Importantly, this
compensatory mechanism increases oxygen demand
on the heart and precludes a vicious cycle that fur-
ther diminishes cardiac output. Paents may be ad-
mied for cardiogenic shock following an acute
event such as a myocardial infarcon or, alternave-
ly, as an acute exacerbaon of chronic heart failure.
Unlike paents with stable, chronic heart failure,
however, the paent in cardiogenic shock experi-
ences rapid changes to which the body is unaccus-
tomed to adapng. For this reason, paents in cardi-
ogenic shock may be parcularly tenuous to man-
age. Paents suering from cardiogenic shock ap-
pear hypotensive, tachypneic, tachycardic, and oen
cool to the extremies from hypoperfusion. During
the acute phase of shock, paents may require vaso-
pressors and inotropes to support blood pressure
and cardiac output. Paents who do not respond
adequately to these treatments may need tempo-
rary mechanical support to augment cardiac output.
Treatable causes of cardiogenic shock such as acute
valve abnormalies, infarcon, or tamponade
should be ruled out immediately.•
Figure 5.4 Cardiomyopathy. From: Blausen.com sta (2014). "Medical gallery of Blausen
Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. [CC BY
3.0 (hps://creavecommons.org/licenses/by/3.0)]

Back to Table of Contents 54
Use the knowledge gained in this chapter and the following scenario to answer the quesons below. When you
are ready, check you answers on p. 69.
You are caring for a paent admied from the cath lab following a bare metal stent placement to the
RCA. The paent has a dressing on his le groin access site following sheath removal.
1. The paent complains of back pain and wants to know when he can sit up in the chair. Based on his
intervenon, you inform him that he must lay at for:
A. 2 hours aer sheath removal
B. 4 hours aer sheath removal
C. 6 hours aer sheath removal
2. Based on the area of his infarct, you ancipate that the paent will receive all of the following
medicaons EXCEPT:
A. ACE Inhibitors
B. Aspirin
C. Beta Blockers
3. Over the course of their admission, the paent develops increased fague and signicant peripheral
edema. Based on the clinical history, you suspect these changes may be due to:
A. Bi-ventricular failure
B. Le Ventricular failure
C. Right ventricular failure
A P P LY Y O U R K N O W L E D G E : CLINICAL CASE STUDY

Back to Table of Contents 55
d v a n c e d T h e r a p i e s a n d D e v i c e s
A
6
Me c ha n ical S u p po r t fo r Ca rd iac Co m p romi s e
Paents experiencing cardiac compromise may
need mechanical support to augment their cardiac
output. This chapter reviews a variety of mechani-
cal assist devices ulized in the CVICU to improve
cardiac output, either temporarily or as permanent
cardiac support. An introducon to device mechan-
ics, nursing sensive acons, and basic trouble-
shoong will be reviewed with each secon.
TEMPORARY PACEMAKERS___________________
Temporary pacemakers are ulized to increase
heart rate and may be inserted for conducon dis-
orders such as heart blocks, rate disorders such as
symptomac bradycardia, or prophylaxis for post-
surgical augmentaon of cardiac output. In the
CVICU, temporary pacing is executed most fre-
quently through the use of epicardial wires placed
directly on the myocardium during surgery. Alterna-
vely, a paent may have pacing wires placed
transvenously, threading either a temp-perm pac-
ing wire or a pacing-capable PA catheter through
the right atrium. In an emergency, transcutaneous
pacing may be ulized.
Nursing Consideraons
When assuming care of a paent with a pacemaker,
nurses should note the mechanism of pacing
(transvenous or epicardial), the part of the heart
being paced (atrial or ventricular), and the mode of
pacing (demand or asynchronous). In a demand
pacing mode, the pacemaker will only pace if the
paent’s rhythm drops below a set threshold. Asyn-
chronous pacing ignores the paent’s underlying
rhythm and should never be used on a paent with
an viable intrinsic rhythm. Doing so may result in an
R-on-T phenomenon that causing lethal arrhythmia.
All temporary pacing wires will be connected to a
temporary pacing box. The replacement baery is
taped to pacer at all mes. Baery changes are
done with shi change at 0700 every day and, for
paents that are pacer dependent, two nurses must
be present. The locaon of the back-up pacemaker
should also be conrmed at shi change. If a pa-
ent is asystolic underneath their paced rhythm, a
back up pacemaker box must be at the bedside
with the same programmed sengs as the primary
pacemaker box.
Underlying rhythm should be checked daily by low-
ering the heart rate seng on the temporary pace-
maker unl the underlying rhythm is revealed. If, at
any point, the paent does not tolerate lowering
the rate seng, the nurse should reset the rate to
the original seng and nofy the provider team..
Underlying rhythm check is done only with a pro-
vider at the bedside for paents with life-
threatening underlying rhythms or severe brady-
cardia. Due to the risk of malposion, paents with
transvenous pacers who are pacer dependent are
on strict bedrest unless a specic order is present
to allow paent to chair or bedside commode.
Troubleshoong
The most common complicaons from temporary

Back to Table of Contents 56
pacing generally fall into two categories: loss of cap-
ture and inappropriate pacemaker sensivity. Loss of
capture occurs when the electrical smuli delivered
by the pacemaker does not result in depolarizaon
of the atria or the ventricle. Failure to capture is not-
ed on EKG by the presence of pacemaker spikes
without corresponding P-waves or QRS complex, de-
pending on the area of the heart being paced. To x
this problem, the nurse must increase the electrical
output from the pacemaker box.
By contrast, sensivity describes the pacemaker’s
ability to detect intrinsic cardiac electrical acvity.
Therefore, inappropriate pacemaker sensivity oc-
curs when the pacemaker does not sense the heart’s
intrinsic electrical acvity correctly. This appears on
EKG as inappropriate pacemaker spikes. If the pace-
maker is too sensive (oversensing), the pacemaker
will inhibit itself inappropriately and underpace the
paent. If the pacemaker is not sensive enough
(undersensing), the pacemaker will overpace the pa-
ent. Both over and under sensing can be dangerous
and require immediate intervenon. Sensivity is
MALFUNCTION CAUSES INTERVENTIONS
Loss of Capture Increased pacing thresholds due to:
• Fluid status changes
• Pericardial eusion
• Electrolyte or metabolic disturbances
• Tissue brosis
Interrupon in pacing system
Dislodged/fractured lead
Ensure connecons secure
Recheck pacing threshold; may need to in-
crease output (mA)
Turn paent
Replace baery
Replace pacemaker
Undersensing Inadequate QRS signal
MI
Fibrosis
Electrolyte disturbance
Bundle Branch Block
Fusion Beat
Baery depleon
Increase sensivity (decrease mV) – allows
pacemaker to more readily see intrinsic cardi-
ac acvity
Correct underlying problem
Oversensing Electromagnec interference
Mypotenal inhibion
T waves outside of the refractory period
Dislodged or fractured lead
Inappropriate sensivity seng
Check pacemaker connecons
Decrease sensivity (increase mV) – pacemak-
er is seeing too much
BOX 6.1 Pacemaker Malfuncons
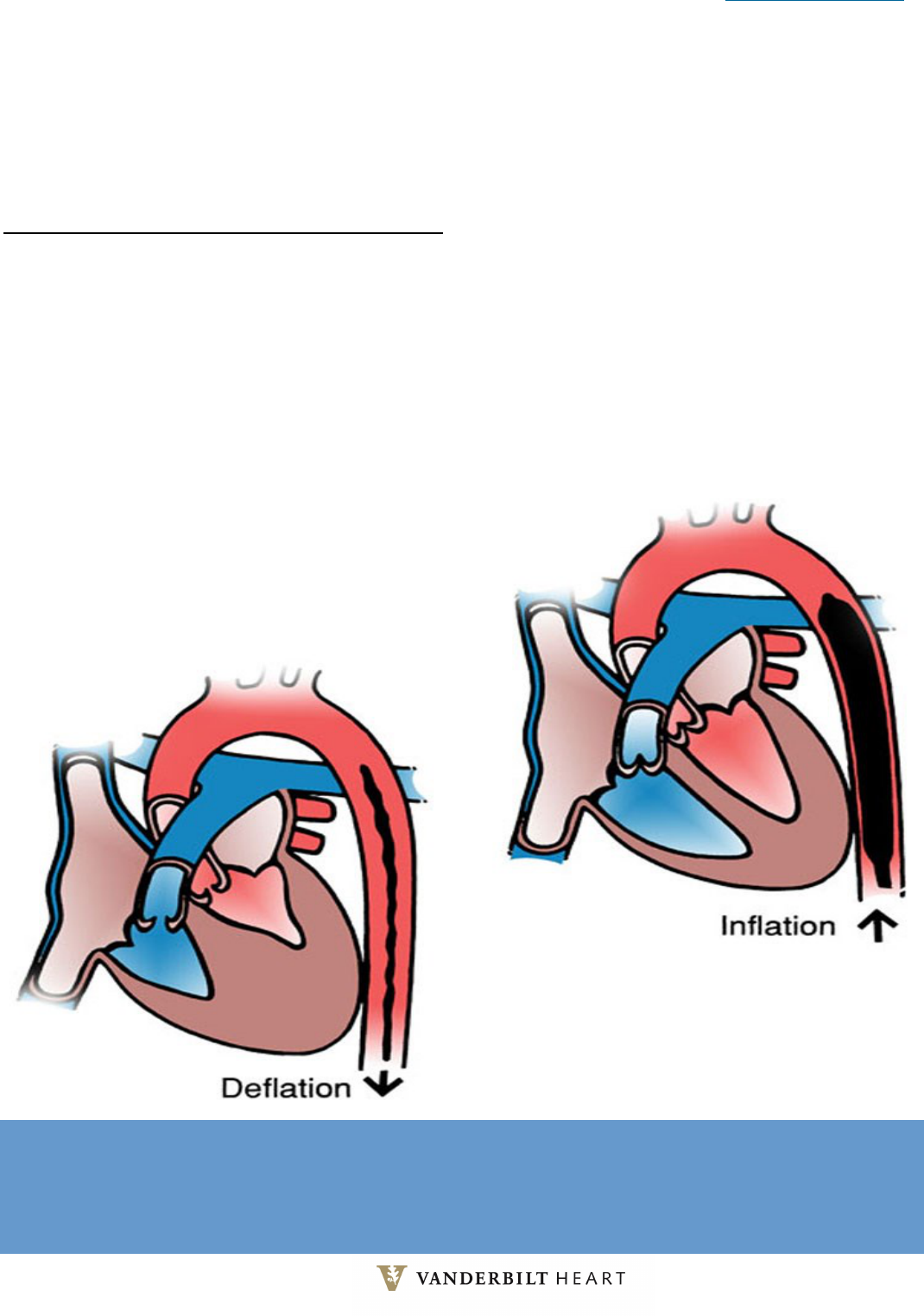
Back to Table of Contents 57
adjusted by manipulang the millivolts (mV) on the
pacemaker box. Increasing the millivolts will de-
crease sensivity and decreasing the millivolts will
increase sensivity. A complete list of causes and
intervenons for loss of capture and inappropriate
sensivity can be found in Box 6.1.
INTRA AORTIC BALLOON PUMP ________________
An intra-aorc balloon pump (IABP) is a device in-
serted through a sheath in the femoral artery that
augments cardiac output, decreases aerload, and
improves myocardial oxygenaon. The balloon is sit-
uated in the aorta just distal to the le subclavian
artery and proximal to the renal arteries. The bal-
loon inates during diastole to displace blood into
the coronary arteries, improving myocardial oxygen
supply. The balloon deates just prior to the next
systole. By deang immediately prior to the next
systolic contracon, the balloon does not allow the
aorta an opportunity to recoil, eecvely decreasing
aerload and myocardial oxygen demand. The me-
chanics of an IABP will provide approximately 1.5 L/
min of addional cardiac output.
Nursing Consideraons
As with any large sheath, nurses should closely mon-
itor the site for bleeding and hematoma formaon.
Paents with a femorally-inserted IABP must remain
on bedrest with the head of their bed elevated no
more than 30 degrees. Nurses should monitor urine
output every hour to ensure that the device has not
migrated distally and obstructed the renal arteries.
Figure 6.1 Intra Aortic Balloon Pump Ination and Deation. Counterpulsation. (Courtesy of MAQUET Cardiovascular,
LLC, Wayne, NJ.) Used with permission (El Sevier, 2019).

Back to Table of Contents 58
Peripheral pulses, including upper extremity pulses,
should be monitored at minimum every two hours
and more frequently as needed. Loss of upper ex-
tremity pulses may indicate that the device has mi-
grated too far into the aorta, obstrucng the subcla-
vian arteries. Loss of pedal pulses may indicate distal
clot showering or obstrucon of peripheral perfusion
by the femoral sheath.
Regarding the balloon and console, nurses should
assess the IABP waveforms every hour to ensure
that the balloon is inang and deang appropri-
ately within the cardiac cycle. When funconing ap-
propriately, the balloon should begin augmenng
diastole at the level of the dicroc notch and deate
such that the assisted diastole is lower than the un-
assisted diastole (Figure 6.2). Assisted and unassist-
ed numbers are documented in eStar every two
hours.
Nurses also should monitor the tubing that supplies
helium to the balloon every hour. If blood is noted in
the tubing, the nurse should immediately turn o
the balloon pump and nofy the provider team that
the balloon has lost its integrity. Once a balloon con-
sole is turned o, the balloon must be removed by a
provider within 30 minutes to reduce the risk of clot
formaon, which may become dislodged during re-
moval. The character of the helium lumen waveform
may also provide addional informaon regarding
balloon funcon and integrity.
Troubleshoong
The most common and easily remedied complicaon
from IABP therapy is improper ming. Nurses may
adjust inaon and deaon intervals as needed to
achieve correct ming of the IABP. Because an IABP
uses predicve algorithms to me balloon inaon
and deaon, balloons may have diculty achieving
correct ming in irregular rhythms. If a paent is in
an irregular rhythm, the nurse should place the bal-
loon in the arrhythmia tracking mode, denoted as “R
-Track” on some consoles. This mode will automa-
cally deate the balloon in response to a premature
QRS complex, ensuring that the balloon is deated
during systole.
In the event of an emergency, the console should be
Figure 6.2 Correct IABP Timing. (Courtesy of MAQUET
Cardiovascular, LLC, Wayne, NJ.) Used with permission (El Sevier 2019)
ALARM INTERVENTION
IABP Disconnected Reconnect IABP tubing and hit “restart”.
Autoll Failure Check helium tank/tubing connecons; ensure tank is open; check helium volume.
Augmentaon Below Set Limit
Check paent’s hemodynamics and treat as needed; If alarm limit inappropriate for
paent, adjust limit.
Rapid Gas Loss
Observe for blood in tubing; If blood not observed, verify tubing connecons and hit
“restart”.
Check IABP Catheter
Check catheter for kink; verify catheter placement in sheath to ensure balloon is not
in sheath; Consider manually inang and deang the balloon.
BOX 6.2 Common Maquet Alarms and Intervenons

Back to Table of Contents 59
placed in pressure trigger mode so that the IABP will
automacally inate and deate in response to
chest compressions. On Maquet consoles, nurses
can change the trigger in semi-auto mode by ad-
jusng the trigger menu on the console. If an Arrow
console is in autopilot mode, the console algorithm
will move directly into pressure trigger if the EKG
cable is pulled from the console. Alternavely, nurs-
es may place the Arrow console in Operator mode
and change the trigger using the trigger menu.
The Arrow IABP console uses a ered alarm system
in which lower priority alarms result in a message-
only display and higher priority alarms result in an
audible alarm. The Arrow console will provide trou-
bleshoong messages related to each alarm and the
alarm will automacally reset once the incing inci-
dent has been resolved. Gas loss and trigger loss
alarms will default the console to “o” and should
be invesgated immediately.
The Maquet console uses only audible alarms. Each
alarm will display an error message on the soware
screen. A summary of common Maquet alarms and
associated intervenons can be found in Box 6.2.
IMPELLA___________________________________
An Impella is a temporary mechanical support de-
vice inserted through a tear-away sheath in the
femoral artery. The distal end of the device is situat-
ed in the le ventricle, pulling blood from the le
ventricle and ejecng it across the aorc valve into
the systemic circulaon (Figure 6.3). Depending on
the specic Impella inserted, the device may pro-
vide 2.5L/min, 3.0L/min, or 5.0L/min of addional
cardiac output. Less commonly, an Impella RP may
be inserted through the femoral vein, sing across
the pulmonic valve with the distal p in the pulmo-
nary artery to provide right ventricular support.
Nursing Consideraons
As with an IABP, the sheath, inseron site, and puls
es distal to the inseron site should be monitored at
roune intervals.
An Impella requires a purge uid of dextrose with
heparin running through the device to prevent
blood from entering the motor housing. For a le
ventricular device, the standard purge uid concen-
traon is 12.5 units/ml. For a right ventricular de-
vice, the standard purge uid concentraon is 50
units/ml. Nurses should monitor and document the
purge uid ow rate and pressure hourly for the
duraon of support.
Nurses should also monitor the quality of the motor
current every hour. Motor current is a measure of
the energy intake of the Impella catheter motor.
The energy intake varies with motor speed and the
pressure dierence between the inlet and outlet
areas of the cannula. Monitoring motor current pro-
vides informaon about the catheter posion rela-
Figure 6.3 Impella for Left Ventricular Support
(From Abiomed®, Inc. [2018]. Impella 5.0® heart pump: For patients
with AMI cardiogenic shock [product brochure]. ABIOMED, Inc.:
Danvers, MA.)

Back to Table of Contents 60
ve to the aorc valve. When posioned correctly,
with the inlet area in the ventricle and the outlet
area in the aorta, the motor current is pulsale be-
cause the pressure dierence between the inlet and
outlet areas changes with the cardiac cycle. When
the inlet and outlet areas are on the same side of
the aorc valve, the motor current will be damp-
ened or at because there is lile or no pressure
dierence between the inlet and outlet areas. Im-
pella RP may demonstrate a sporadic and damp-
ened motor current since it crosses two valves on
the venous side of the heart. This should be noted
as a normal variaon for right-sided support.
Lastly, nurses should document device ow rate and
power level, commonly called P-level, hourly. The
greater the P-level, the more support the device is
providing. Impella P-levels range from P-2 to P-8.
Troubleshoong
If a paent with an Impella codes, nurses should
perform CPR and debrillaon as needed. AbioMed
recommends turning down ow to P-2 during CPR.
Nurses should note, however, that placement moni-
toring and ow calculaons will not be accurate dur-
ing CPR. Once ROSC is achieved, device placement
should be checked immediately with transthoracic
echo.
The most common Impella alarm is a sucon alarm.
A sucon alarm is most commonly caused by
hypovolemia. If a sucon alarm is noted, the nurse
should turn the P-level down unl the sucon is re-
leased and nofy the team. If the CVP is below 10
mmHg, the nurse should also request an order for a
bolus to uid resuscitate the paent. However, if
the paent is adequately uid resuscitated, the right
ventricle should be invesgated via transthoracic
echo.
In the event that an Impella posion alarm sounds,
nurses should conrm the posion by assessing the
motor current waveform against the Impella place-
ment signal waveform as well as the placement
markers on the Tuohy-Borst valve where the Impella
exits the paent’s groin. If the placement waveform
or cenmeter markings are noted to be dierent
from the paent’s baseline, the provider team
should be noed immediately and Impella place-
ment should be re-evaluated with a transthoracic
echo. Placement alarms will not sound with an Im-
pella RP device and placement should instead be
veried with chest x-ray.
CENTRIMAG________________________________
Centrimag is a short-term centrifugal support device
for paents in acute heart failure. The device de-
creases ventricular workload by bypassing the
aected area of the heart, improving hemodynamic
condions to opmize myocardial recovery. Cen-
trimag may be ulized to support le ventricular,
right ventricular, or bi-ventricular failure. A cen-
trimag can provide up to 9 L/min of support.
To support the le ventricle, a drainage cannula will
be placed in the le atrium or ventricle with a re-
turn to the aorta. Right-sided support is used less
frequently and is most commonly ulized to support
right ventricular failure following transplant, transi-
ent pulmonary hypertension, or right-sided infarc-
on. To support the right ventricle, a drainage can-
nula will be placed in the right atrium with a return
cannula to the pulmonary artery.
Nursing Consideraons
Nurses should monitor the cannulas to ensure that
there are no kinks and that the cannulas have not
moved. Centrimag cannulas are almost exclusively
placed in the OR via direct cardiotomy and should
be treated with extreme care. Paents with Cen-
trimag support should be reposioned every two
hours but perfusionist and/or provider must be pre-
sent for any addional mobilizaon.
Device ow is controlled by the RPMs set by the
provider as well as the paent’s intrinsic preload

Back to Table of Contents 61
and aerload. Centrimag RPMs are typically set be-
tween 3,000-4,000 to achieve a ow of 3-4 L/min,
depending on paent size and ventricular funcon.
Pump ow and speed should be documented every
hour for duraon of therapy. In the instance of bi-
ventricular failure, nurses should closely monitor
that right ventricular ow does not exceed le ven-
tricular ow, which could cause pulmonary edema
and worsen the paent’s clinical condion.
Hypertension may decrease Centrimag ow rate at
a set RPM. Nurses should trate pressors to achieve
a systemic pressure of approximately 90/70 with
mean of 70-80 mmHg. Note that the arterial line in
paents with a Centrimag will oen appear at due
to non-pulsale ow from the device.
Paents receiving Centrimag support are also at risk
for clong and hemolysis. Nurses should monitor
coagulaon labs, including PTT or ACT, at the discre-
on of the provider team. Nurses should also ob-
serve device tubing for brin strands or clot every
hour, paying close aenon to angles and connec-
on points since these areas are parcularly at risk
for clot formaon. To prevent clot formaon around
the ow probe, the ow probe should be moved at
least once per shi. Any new or changed clot should
be reported immediately to the provider team.
Troubleshoong
Because the Centrimag pump is preload-dependent,
nurses should closely monitor CVP and work with
their provider teams to maintain a CVP of 10-15
mmHg. If the pump does not have enough preload,
the nurse may observe passive swinging of the
drainage cannula, commonly referred to as chug-
ging or chaer. This should be reported to provid-
ers immediately since the provider may decrease
the device speed temporarily to resolve the issue
unl the paent’s volume status can be ad-
dressed.
If the primary pump ceases to funcon, the nurse
will need to press the sta assist buon and switch
the pump head to the back up controller immedi-
ately. When switching to a back up controller, the
nurse should always clamp the oulow tubing rst
and then press and hold stop buon on the primary
controller. Stopping the pump without clamping will
allow retrograde ow. Once the pump head is es-
tablished in the back-up pump motor housing, the
device should be turned on and RPMs increased to
10000 prior to unclamping the oulow tubing and
returning the device to the set speed.
Although air entrainment is uncommon in a Cen-
trimag device, it is always an emergency. If air is
noted in the device, perfusion and the provider
team should be noed immediately.
Extra-corporeal Membrane Oxygenaon (ECMO)__
Extra-Corporeal Membrane Oxygenaon (ECMO) is
a treatment plaorm used to support paents in the
seng of severe heart and/or lung dysfuncon.
ECMO is used to support paents whose organ sys-
tems should recover such as those suering from
pneumonia, ARDS, Takotsubo cardiomyopathy, or
acute rejecon following heart or lung transplant. It
can also be used as a bridge therapy for those in end
-stage heart or lung disease to either durable me-
chanical support (VAD) or transplant. ECMO is not a
curave therapy. It does, however, allow crically ill
At least two specialized tubing clamps
should be hanging on the Centrimag and
ECMO consoles at all mes. In the rare
instance that a nurse needs to clamp out
the circuit to switch to the back-up
controller or hand crank, these clamps
will not harm the tubing integrity.
PRECEPTOR PEARLS

Back to Table of Contents 62
paents and their provider team me to explore
their underlying disease dierenal to create a
treatment plan.
There are two types of ECMO support. Veno-Arterial
(VA) ECMO supports a paent’s hemodynamics and
oxygenaon by bypassing both the cardiac and pul-
monary circulaon. By contrast, Veno-Venous (VV)
ECMO supports a paent’s oxygenaon only. To
cannulate a paent for ECMO, a large bore cannula
is placed in a primary vein to drain blood into the
ECMO circuit. A centrifugal pump pulls blood from
the venous system using negave sucon. The
blood comes out of the centrifugal pump in a posi-
ve pressure that then pushes blood through an ar-
cial lung (oxygenator). Inside the oxygenator, ox-
ygen is picked up and CO2 is cleared just like in a
paent’s nave lungs. Blood is then pushed back to
the paent and reinfused through a cannula either
into their venous system (VV ECMO) or to the arteri-
al system (VA ECMO). ECMO always drains blood
from a vein – where the blood is returned deter-
mines what kind of ECMO the paent is on and
which organ system is being supported or bypassed.
Nursing Consideraons
Well-supported paents on ECMO may seem very
stable; however, they are in fact among the sickest
paents in the hospital. As such, it’s important to
remember than an ECMO-trained nurse must be
available to watch the paent at all mes, even if
the paent’s primary nurses only ancipates being
away from the bedside for a short period of me.
Nurses should be very cauous with the cannulas,
circuity and pump, encouraging families and other
sta to avoid close proximity with pump compo-
nents in order to protect the circuit.
Cannula site dressing changes are done with the
same frequency and manner as central line dressing
changes. It is important not to use alcohol-based
products because these products will break down
the polymers in the ECMO circuitry and cause it to
become brile. Central cannulaon dressing chang-
es are done per the provider’s discreon.
All ECMO paents, including open chest central can-
nulaon, should be reposioned at minimum every
two hours to redistribute pressure and promote skin
Figure 6.4 A, Peripheral venoarterial extracorporeal membrane oxygenation [ECMO] standard cannulation and
circuit. B, Peripheral bicaval cannulation venovenous ECMO standard cannulation and circuit. (From Kaplan, J.A. and others
[Eds.]. [2017]. Kaplan’s cardiac anesthesia for cardiac and noncardiac surgery [7th ed.]. Philadelphia: Elsevier.)

Back to Table of Contents 63
integrity. As with Centrimag, addional mobility of
ECMO paents should be conducted only with a
perfusionist present. On rare occasions, a paent
may be deemed too unstable for reposioning. In
this case, and aending physician must enter a
“Do Not Turn” order. This order must be renewed
every 12 hours.
Troubleshoong
Air entrainment will cause the ECMO pump to
clamp and forego supporng the paent. As lile
as 0.3 ml of air may cause the pump to clamp. To
prevent air entrainment, it is important that any ve-
nous access lines including central lines, manifolds,
peripheral IVs, and vascaths have a clave. Extreme
cauon should be used when ushing to ensure that
there is no air in the syringe or IV lines. Due to the
high risk of air entrainment, free ow tubing should
be avoided on ECMO paents.
If something happens to cause the ECMO pump to
stop, an ECMO paent will likely become unstable
very quickly. The nurse should press the sta assist
alarm and nofy perfusion using the perfusion pager
immediately, supporng the paent’s hemodynam-
ics and oxygenaon status unl addional help ar-
rives. In the event that the ECMO pump has lost
power, the nurse should clamp the circuit and tran-
sion pump head to hand crank housing and crank
to desired hemodynamic or oxygenaon goal. Any
addional troubleshoong will be executed by the
provider and perfusion team upon their arrival.
Le Ventricular Assist Device (LVAD)____________
Le ventricular assist devices (LVADs) provide dura-
ble mechanical support to the le ventricle that, un-
like many other devices, allow paents to be dis-
charged from the hospital. Vanderbilt supports
three types of LVADs: Heartmate II, Heartmate III,
and Heartware. All devices are approved as a bridge
to heart transplant or as desnaon therapy.
The Heartmate II pump is an axial ow pump
anastamosed to the apex of the le ventricle and
the ascending aorta. Hearmate II speed ranges from
6,000-15000 rpms (average 9,600 rpms) to provide
3-10 L/min of support. The HMII operates in a xed
speed mode only, which maintains a connuous,
constant ow. The inial speed is set in the oper-
ang room at approximately 6,000 rpm and gradual-
ly increased in increments of 200 rpm with TEE ob-
servaon unl the desired ow is achieved. Adjust-
ments can be made by members of the VAD team
using the System Monitor as needed, depending on
the paent’s clinical status. When the pump is func-
oning appropriately, an increase in speed (rpms)
will increase ow while a decrease in speed (rpms)
will decrease ow. As speed increases, device power
will also increase.
By contrast, Heartmate III and HeartWare devices
are centrifugal pumps anastamosed to the le ven-
tricle and aorta. The Heartmate III speed ranges
from 3,000-9,000 rpms (average between 4800 and
5800) to achieve a ow of 3-10 L/min. Unlike Heart-
mate II, Heartmate III has generates an arcial
pulse every two seconds by dropping the speed
2000 rpms above and below the set speed. These
speed changes will not be visible on the system
monitor, but may be assessed on the paent’s arte-
rial waveform. HeartWare devices are xed ow de-
vices that deliver a constant speed between 1800
Strict air precauons should be ulized
for Centrimag and ECMO devices. This
means that no free-ow tubing is hung ,
claves on every access port, and even
small bubbles of air must be
meculously removed from any
medicaon prior to being given.
PRECEPTOR PEARLS

Back to Table of Contents 64
and 4,000 rpms (average 2400-3200 rpms) to
achieve the desired ow.
Nursing Consideraons
Because LVADs are connuous ow devices, narrow
pulse pressures are a normal variaon in this paent
populaon. Peripheral pulses may not be palpable
and non-invasive blood pressures will be assessed
using a doppler. Vasoacve drips will be trated to
achieve mean arterial pressure (MAP) since systolic
blood pressures are unreliable in VAD paents. With
connuous ow devices, a pulse pressure greater
than 30 mmHg may imply inadequate LV unloading,
and the LVAD speed may need to be increased. If a
pulse pressure greater than 30 mmHg is assessed,
the provider team should be noed. All speed
changes require an MD order. Device parameters
may only be adjusted by an aending physician,
heart failure aending, or surgeon.
All LVAD devices are preload dependent and aer-
load sensive. As such, nurses should ensure that
paents are adequately uid resuscitated, maintain-
ing a CVP between 10 and 15 mmHg. CVP greater
than 20 should be reported to the provider team. To
manage aerload, nurses should maintain a MAP of
60-80 mmHg. Doppled MAPs are correlated with the
paent’s arterial line at minimum every shi. When
the paent’s arterial line is removed, doppled MAPs
are recorded every two hours. MAPs greater than
90 should be reported to the provider team since
increased MAP may decrease device ows.
Pump ow, speed, power, and pulsality index are
assessed every hour and recorded in eStar. Pulsali-
ty index (PI) is the magnitude of ow pulses through
the pump. During le ventricular systole, the in-
crease in ventricular pressure causes an increase in
pump ow. Higher values indicate more ventricular
lling and higher nave pulsality, indicang that
the pump is providing less support to the ventricle.
Lower values indicate less ventricular lling and low-
er nave pulsality, indicang that the pump is
providing greater support to the le ventricle. In
Heartmate devices, PI will be provided numerically
on the device screen. In HeartWare devices, the PI is
calculated by subtracng the trough of the pulsali-
ty waveform from the peak of the pulsality wave-
form on the device monitor.
Driveline infecon is a major cause of morbidity and
mortality in VAD paents. For this reason, mecu-
lous driveline care is a nursing priority. As a stand-
ard, operave dressings remain in place for the rst
72 hours. Gauze dressings are applied and changed
daily on post-operave days three through seven. If
site drainage is minimal and conversion is approved
by a VAD Coordinator, Centurion dressings begin on
POD 8. Centurion dressings are changed every three
days and PRN on the implant admission. Nurses
should note that Centurion dressing change fre-
quency during readmissions may vary depending on
the stage of healing, site appearance, and/or home
dressing change schedules previously established by
Figure 6.5 Implanted HeartWare® Ventricular Assist
Device pump. (Courtesy of HeartWare, Inc.,
Framingham, MA.) (El Sevier, 2019)

Back to Table of Contents 65
the paents and their VAD Coordinators.. Centurion
dressings are changed on any day that the paent
showers irrespecve of standard change schedules.
Troubleshoong
In the event of a cardiopulmonary arrest, chest com-
pressions will not be performed without an order
from an aending physician to decrease the risk of
device dislodgement causing a surgical emergency.
In the event of a cardiac arrest:, the nurse should
rst conrm that the power supply and system con-
troller are operaonal. Next the nurse should deb-
rillate and use medicaons per ACLS protocol.
A sucon event occurs when ows drop below 2.5
L/min and may be due to ventricular collapse or in-
ow occlusion. Ventricular collapse occurs when a
connuous ow VAD aempts to pump more blood
from the le ventricle than is available, resulng in
considerable reducon in ventricular volume. Le
ventricular collapse can be the result of clinical
events aecng le ventricular preload, including
hypovolemia (bleeding), right heart failure, arrhyth-
mia or pulmonary embolus. An inow occlusion oc-
curs when the inow cannula is obstructed, causing
a sucon condion. Temporary inow obstrucon
may also occur as a result of surgical posioning,
paent posion or during straining (valsalva). If a
paent experiences a sucon event, the nurse
should immediately nofy the provider team, sup-
port the paent’s hemodynamics, and ancipate
treatment of the root cause of the event based on
the paent’s clinical picture. To provide the nurse
and audible alert of a sucon event, the nurse
should ensure that the sucon alarm is on at the
beginning of each shi.
Other LVAD complicaons may include: PI events,
pump thrombus, right ventricular failure, and ar-
rhythmias. A PI event is characterized by a PI less
than 2 with a correlave drop in device ows and
may indicate that more cardiac support is indicat-
ed. Pump thrombus is characterized by decreased
ows with a correlave increase in pump power and
may indicate the need for further evaluaon and
thrombolyc therapy. Right ventricular failure in the
LVAD paent presents similarly to right ventricular
failure in the paent without mechanical circulatory
support. However, if severe, right ventricular failure
may result in decreased LVAD ow due to inade-
quate le ventricular preload. Arrhythmias are com-
mon in the LVAD paent populaon and should be
treated using the same algorithms ulized in pa-
ents without mechanical circulatory support. Im-
portantly, due to the nature of connuous ow de-
vices, LVAD paents generally tolerate arrhythmias
beer than paents without a connuous ow de-
vice. Treatment type and urgency are determined
by assessing paent clinical status. All new arrhyth-
mias should be reported immediately to the provid-
er team, regardless of the paent’s clinical status.
TOTAL ARTIFICIAL HEART______________________
Total arcial heart is a durable mechanical support
device approved as a bridge to transplant for pa-
ents with biventricular failure, le ventricular
thrombus, or malignant arrhythmias that preclude
LVAD implantaon. To implant the device, all four
nave valves and both ventricles are removed and
replaced with two mechanical ventricles and four
mechanical valves. Blood is moved through the de-
LVAD paents generally tolerate
arrhythmias beer than paents without
connuous ow devices. Paent
assessment is paramount to determine
what intervenon is most appropriate
and the degree of urgency that this
intervenon requires.
PRECEPTOR PEARLS

Back to Table of Contents 66
-vice by inang and deang diaphragms within
the ventricles via pneumac drivelines that connect
to the device console.
Syncardia recommends adjusng device parameters
to achieve paral device ll and full ejecon of
blood from the device. Paral ll is dened as bi-
ventricular ll volumes of 50-60 ml for a 70 ml de-
vice, allowing the device to funcon eecvely with
normal variaons in venous return to the heart. Full
ejecon is determined by observing a secondary rise
in pressure, known as the full eject ag, on the pres-
sure waveform (Figure 6.7, A). Full ejecon ensures
that clot does not form within the ventricles due to
blood stasis. Providers may adjust the device beat
rate and percent systole as well pneumac drive
and vacuum pressures to achieve these goals. An
increase in beat rate, or decrease in vacuum pres-
sure will decrease ventricular ll volume. An in-
crease in pneumac drive pressure will increase the
force by which blood is ejected from the ventricles.
Adjustment of device parameters from baseline
should always be viewed as a temporizing measure
and the paent’s clinical picture must always be tak-
en into account. As with LVADs, only aending phy-
sicians, heart failure aendings, or a surgeon may
adjust device parameters.
Nursing Consideraons
Because device implantaon requires removal of
the nave ventricles, the paent will not have an
QRS complex on EKG. For this reason, EKG is not
monitored on paents with a Total Arcial Heart.
Unlike LVADs and other connuous ow devices,
the Total Arcial Heart is pulsale and will produce
a blood pressure with a pulse pressure within the
normal range. For this reason, automac blood
pressure cus will produce a reliable blood pressure
and doppled pressures are not required.
Nurses should assess device output and ll for both
ventricles every at least every hour and PRN as pa-
ent condion warrants. Device beat rate; percent
systole; le and right drive pressures; and le and
right vacuum pressures are documented every hour.
In addion to these parameters, nurses should mon-
itor the pressure, ow, and average cardiac output
waveforms hourly (Figure 6.7).
The pressure waveform measures the pressure of
air in the pneumac driveline required to generate
systole. A normal pressure waveform will demon-
strate a secondary rise in pressure, known as the full
eject, ag (Figure 6.7, A). The ow waveform
demonstrates the ow of air in L/min moving out of
the pneumac drivelines, deang the ventricular
diaphragms. A normal ow waveform should
demonstrate consistent ow of air from the ventri-
cles throughout diastole, depicted as bilateral paral-
lel lines following the opening of the inow valves
into the heart (Figure 6.7, B). Any change from base-
line should be reported to the provider team.
Figure 6.6 Total Articial Heart. Used with permission.
Syncardia, 2019.

Back to Table of Contents 67
Troubleshoong
In the event of an emergency, CPR, debrillaon,
and ACLS protocols should not be ulized. If a pa-
ent with a Total Arcial Heart is found without a
pulse, the nurse should rst assess that the driveline
is intact and plugged into the console. If this does
not resolve the issue, the nurse should ensure that
the console is on and funconing appropriately,
switching to the back up console if required. If this
does not resolve the issue, the nurse should switch
to the hand pump, assist with respiraons, and pre-
pare to administer a 1L normal saline bolus. If a pa-
ent with a Total Arcial Heart is found unrespon-
sive with a pulse, the nurse should support respira-
ons and assess for non-cardiac eologies of the
change in level of consciousness such as hypoglyce-
mia, stroke, or hypoxia.
As with other mechanical circulatory devices, the
Total Arcial Heart is aerload sensive. Systemic
or pulmonary hypertension may prohibit the device
from ejecng eecvely. If a full eject ag is lost on
the le pressure waveform, the nurse should rst
assess the paent’s blood pressure and trate medi-
caons to maintain a systolic blood pressure be-
tween 90 and 140 mmHg. The provider may choose
to increase the pneumac drive pressure to improve
ejecon unl normotension is achieved. If a full
eject ag is lost on the right pressure waveform,
pulmonary vasodilators may be considered.
Cardiac tamponade is a major complicaon in the
immediate postoperave phase following implanta-
on. Cardiac tamponade is characterized by declin-
ing cardiac output on the cardiac output graph
(Figure 6.7, C) that coincides with decreased ll vol-
umes and diastolic ow. If the obstrucon is in the
inow tract, the paent will present with a charac-
terisc rise in CVP. If the obstrucon is in the
oulow tract, a loss of full eject may be observed.
Suspicion of tamponade is a surgical emergency and
should be reported immediately to the provider
team. •
Figure 6.7 Expected Waveforms for Total Articial Heart. Used with permission. Syncardia, 2019. A. The pressure
waveform depicts the pressure within the pneumatic drivelines during systole. B. The ow waveform depicts the ow of air
out of the pneumatic drivelines during diastole. C. The cardiac output graph depicts the average cardiac output over time.
A.
B.
C.

Back to Table of Contents 68
Use the knowledge gained in this chapter and the following scenario to answer the quesons below. When you
are ready, check you answers on p. 70.
You assume care of a paent with a le-sided Impella CP placed through the le femoral artery. The
plan is to send this paent for a HeartMate III later in the shi. The paent has a PA cath displaying
the following informaon: PA pressure 35/22 and CVP 5 mmHg.
1. While performing your morning assessment, the Impella sounds a sucon alarm. Your next acon is
to:
A. Turn o the console to prevent hemolysis
B. Turn down the P-Level unl the sucon alarm resolves
C. Press the sta assist buon and wait for help to arrive
2. The sucon alarm resolves. In response to this event, you ancipate the team will:
A. Order a 500 ml uid bolus
B. Pull the Impella within 30 minutes of the console being turned o
C. Increase epinephrine to achieve hemodynamic targets
3. Following implantaon of the paent’s HeartMate III, the paent enters in to V-Tach. The paent’s
MAPs drop from 65 to 40. Your next acon is to:
A. Debrillate the paent at 200 J
B. Iniate chest compressions at 120 bpm
C. Push 1 mg epinephrine and obtain an order for lidocaine
A P P LY Y O U R K N O W L E D G E : CLINICAL CASE STUDY

Back to Table of Contents 69
Chapter 2
1. A. Raonale: The normal CVP is 2-8 mmHg. A CVP of 18 is elevated and thus represents volume overload.
2. B. Raonale: Although an index of 1.7 may be normal for a paent in cardiogenic shock, this should be
immediately reported to the provider because it is below the threshold for reportable values and requires
treatment. While you may evaluate the paent’s PVR and SVR, this is not the most urgent acon.
3. A. Raonale: The paent is exhibing respiratory acidosis. To relieve this condion, the minute venla-
on should be increased by increasing the respiratory rate.
Chapter 3
1. B. Raonale: Milrionone will decrease aerload (SVR) and improve cardiac contraclity, thus improving
the cardiac index.
2. C. Raonale: Levophed is an alpha agonist and will improve blood pressure by increasing SVR.
3. A. Raonale: Epinephrine is an inotrope. Decreasing an inotrope will decrease the paent’s cardiac index.
An index below 2 should be reported to the provider team.
Chapter 4
1. B. Raonale: The goal to extubaon for post-cardiotomy paents is 6 hours. The other answers reect
appropriate intervenons for the rst hour aer surgery.
2. C. Raonale: All post-cardiotomy paents are at increased risk of hyperglycemia and acute kidney injury,
however, valve replacement is associated with a higher risk of embolic, ischemic stroke.
3. C. Raonale: A potassium less than three requires nocaon of the provider team and treatment using
the nurse-driven replacement protocol. The ABG does not require venlatory intervenon. Nurses must
contact a provider to obtain an order for blood.
Chapter 5
1. C. Raonale: A paent must remain at for 6 hours aer sheath removal if an intervenon is performed
in the cath lab. If the paent does not receive intervenon in the cath lab, they must remain at for two
hours aer sheath removal
.
C l i n i c a l C a s e S t u d i e s :
A n s w e r s a n d R a t i o n a l e s

Back to Table of Contents 70
2. A. Raonale: Although the paent may have other indicaon to receive an ACE inhibitor, ACE inhibitors
are indicated for le sided infarcons only in order to prevent ventricular remodeling. Aspirin and beta
blockers are indicated for both right and le-sided infarcons.
3. C. Raonale: The symptoms described are indicave of right ventricular failure, which is consistent with
the paent’s presenng infarcon.
Chapter 6
1. B. Raonale: If a sucon alarm sounds, the nurse should immediately turn down the P-level to relieve
the sucon and support the paents hemodynamics as needed. The console should not be turned o
unless therapy is about to be disconnued. While the nurse may choose to hit the sta assist buon,
immediate measures must be taken while the nurse is in the paent room.
2. A. Raonale: Sucon alarms are most commonly caused by hypovolemia. The nurse should ancipate
a uid bolus is needed since the paent’s CVP is less than 10 mmHg. Increased inotropic support
would only be indicated if right ventricular failure was suspected. Turning o the console is not indicat-
ed for a sucon alarm.
3. A. Raonale: Debrillaon is the appropriate intervenon for V-Tach. CPR is contraindicated in paent
with an LVAD, although all other components of ACLS protocol are followed. Per ACLS protocol, deb-
rillaon is the priority intervenon for V-Tach. Epinephrine is only indicated aer the second shock in
pulseless V-Tach.

Back to Table of Contents 71
R e f e r e n c e s
American Heart Associaon. (2018). Adult advanced cardiovascular life support: Web-based integrated
2018 & 2018 American Heart Associaon guidelines for CPR and ECC. Retrieved from: hps://eccguidelines.heart.org
index.php/circulaon/cpr-ecc-guidelines-2/part-7-adult-advanced-cardiovascular-life-support/
American Nurses Credenaling Center. (2011). Magnet recognion program: a program overview.
Retrieved from: hp://nursecredenaling.org/Documents/Magnet/MagOverview-92011.pdf
Boysen, P.G. (2013). Just culture: A foundaon for balanced accountability and paent safety. Retreived
From: hps://www.ncbi.nlm.nih.gov/pmc/arcles/PMC3776518/
Colucci,W.S. (2018). Hydralazine plus nitrate therapy in paents with heart failure with reduced ejecon
fracon. Retrieved from: hp://www.uptodate.com.
Cooper, L.T., Mckenna, W.J., and Yeon, S.B. (2019). Denion and classicaon of the cardiomyopathies. Retrieved from:
hps://www.uptodate.com/contents/denion-and-classicaon-of-the-cardiomyopathies
Gaasch, W.H., & Zoghbi, W.A. (2017). Overview of the management of paents with prosthec heart
valves. Retrieved from: hps://www.uptodate.com/contents/overview-of-the-management-of-paents-with-
prosthec-heart-valves?search=overview-of-the-management-of-paents-with-prothec-heart-
valves&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
Hardin, S.R., & Kaplow, R. (2020). Cardiac surgery essenals for crical care nursing (3rd ed.). Bulington, MA: Jones and Bartle
Learning
Kashou, A. & Kashou, H. (2017). Physiology, Sinoatrial Node (SA Node). Retrieved February 9, 2018,
From: hps://www.ncbi.nlm.nih.gov/books/NBK459238/
Krako, L.R. (2017). Diagnosis and treatment of hypertension. In V. Fuster, R.A. Harrington, J. Narula. &
J. Eapen (eds.), Hurst’s the heart (14
th
ed.). Retrieved from: hp://accessmedicine.mhmedical.com/content.aspx
bookid=2046&seconid=155632214
Nadim, M.K., Forni, L.G., Bihorac, A., Hobson, C., Koyner, J.L., Shaw, A., … Kellum, J.A. (2018). Cardiac
and vascular surgery-associated acute kidney injury: The 20
th
Internaonal Consensus Conference in the ADQI (Acute
Disease Quality Iniave) Group. Journal of the American Heart Associaon, 7(11), e008834. doi: 10.1161/
JAHA.118.008834
Yancy C.W., Jessup M., Bozkurt B., Butler, J., Casey, C.E., Drazner, M.H. et al. (2013). 2013 ACCF/AHA
guideline for the management of heart failure: a report of the American College of Cardiology
Foundaon/American Heart Associaon Task Force on pracce guidelines. Circulaon.
128(16):e240-e327. doi: 10.1161/CIR.0b013e31829e8776





