
ZymoBIOMICS™ DNA Miniprep Kit
DNA for microbiome or metagenome analyses
Highlights
• Validated Unbiased for Microbiome Measurements: Unbiased
cellular lysis validated using the ZymoBIOMICS Microbial Community
Standard.
• Inhibitor-Free DNA from Any Sample: Isolate ultra-pure DNA ready
for any downstream application.
• Certified Low Bioburden: Boost your detection limit for low
abundance microbes.
• Simple Workflow: Simply bead-beat sample, purify via spin-column,
and filter to remove PCR inhibitors. No precipitation or lengthy
incubations!
Catalog Numbers:
D4300T, D4300, D4304
Scan with your smart-phone camera to
view the online protocol/video.

Table of Contents
Product Contents ........................................... 01
Specifications................................................. 02
Product Description ....................................... 03
Protocol .......................................................... 06
Appendices .................................................... 08
Sample Collection ............................................. 08
Application Notes. ............................................. 10
Standardize Sample Preparation with ZymoBIOMICS
Microbial Standards ........................................... 16
Optimized Lysis Protocols for Bead-Beating ............ 19
Troubleshooting ............................................. 20
Ordering Information ..................................... 22
Guarantee ....................................................... 25
INSTRUCTION MANUAL Ver.1.5.4 Revised on: 5/11/2023

1
Product Contents
ZymoBIOMICS™ DNA
Miniprep Kit
D4300T
(5 Preps.)
D4300
(50 Preps.)
D4304
(50 Preps.)
Storage
Temperature
ZR BashingBead™ Lysis
Tubes (0.1 & 0.5 mm)
5
50
-
Room Temp.
ZymoBIOMICS™ Lysis
Solution
4 ml
40 ml
-
Room Temp.
ZymoBIOMICS™ DNA
Binding Buffer
1
6 ml
100 ml
100 ml
Room Temp.
ZymoBIOMICS™ DNA Wash
Buffer 1
2 ml
50 ml
50 ml
Room Temp.
ZymoBIOMICS™ DNA Wash
Buffer 2
5 ml
60 ml
60 ml
Room Temp.
ZymoBIOMICS™
DNase/RNase Free Water
1 ml
10 ml
10 ml
Room Temp.
ZymoBIOMICS™ HRC Prep
Solution
3 ml
30 ml
30 ml
Room Temp.
Zymo-Spin™ III-F Filters
5
50
50
Room Temp.
Zymo-Spin™ III-HRC Filters
5
50
50
Room Temp.
Zymo-Spin™ IICR Columns
5
50
50
Room Temp.
Collection Tubes
20
200
200
Room Temp.
Instruction Manual
1
1
1
-
1
See endospore lysis efficiency data in Appendix B.

2
Specifications
• Sample Sources – Bacterial (including endospores)
1
, fungal,
protozoan, algal, viral, mitochondrial, and host DNA is efficiently
isolated from ≤ 200 mg of mammalian feces, ≤ 250 mg soil, and
50 – 100 mg (wet weight) of bacterial/fungal cells
2
, biofilms, and
water
3
.
• Bead Beating System – The innovative ZymoBIOMICS™ lysis
system enables complete homogenization/disruption of the
microbial cells walls and accurate microbial DNA analysis, free of
bias. To ensure unbiased lysis, calibration of each bead-beating
device is recommended by using the ZymoBIOMICS™ Microbial
Community Standard (see Appendix C).
• DNA Purity – High quality, inhibitor-free DNA is eluted with
ZymoBIOMICS™ DNase/RNase Free Water and is suitable for all
downstream applications including PCR and Next-Generation
Sequencing.
• DNA Integrity – On average, post bead beating, genomic DNA is
between 15-20 kb depending on the initial quality of the sample,
making it amenable to Next-Generation Sequencing platforms
requiring high molecular weight DNA. For optimal DNA integrity,
collect samples in DNA/RNA Shield™
4
.
• DNA Recovery – Up to 25 µg total DNA can be eluted into 100 µl
(50 µl minimum).
• Bioburden – A single preparation is guaranteed to contain less
than 3 bacterial genomic copies per µl of eluate as determined by
quantitative amplification of the 16S rRNA gene when eluted using
100 µl water. Individual components (sold separately) are not
certified low-bioburden.
• Equipment – Microcentrifuge, Vortex Genie
®
, high speed cell
disrupter (recommended).
1
See endospore lysis efficiency data in Appendix B.
2
This equates to approximately 10
9
bacterial cells and 10
8
yeast cells.
3
For water samples, filter using desired filter (not provided). Cut the filter into small pieces and place into ZR
BashingBead™ Lysis Tube (0.1 & 0.5 mm). Alternatively, up to 250 µl water can be processed directly.
4
DNA/RNA Shield™ provides an accurate molecular signature of the sample at the time of collection by reserving
nucleic acids at ambient temperature and inactivating organisms including infectious agents (see Appendix A).

3
Product Description
The ZymoBIOMICS™ DNA Miniprep Kit is designed for purifying DNA
from a wide array of sample inputs (e.g. feces, soil, water, biofilms, etc.),
that is immediately ready for microbiome or metagenome analyses. The
ZymoBIOMICS™ innovative lysis system eliminates bias associated with
unequal lysis efficiencies
1
of different organisms (e.g. Gram-
negative/positive bacteria including endospores
2
, fungi, protozoans,
algae, etc.), making it ideal for microbial community profiling. Unbiased
mechanical lysis of tough microbes is achieved by bead beating with the
innovative ultra-high density BashingBeads™ and validated using the
ZymoBIOMICS™ Microbial Community Standard
3
, as shown in Figure 3.
In addition, the ZymoBIOMICS™ DNA Miniprep Kit is equipped with Zymo
Research’s proprietary OneStep™ PCR Inhibitor Removal technology
enabling PCR from the most PCR prohibitive environmental samples rich
in humic and fulvic acids, tannins, melanin, and other polyphenolic
compounds. Coupling state-of-the-art lysis technology with Zymo-Spin™
Technology results in superior yields of ultra-pure DNA ideal for all
downstream applications including PCR, arrays, 16S rRNA gene
sequencing, and shotgun sequencing
4
.
Innovation. Pure & Simple.™
1
Chemical, enzymatic, and inferior lysis matrices (beads) lead to unrealistic representation of organisms in
downstream metagenomic analyses that is not reflective of actual abundance.
2
See endospore lysis efficiency data in Appendix B.
3
For more information on the ZymoBIOMICS™ Microbial Community Standard (D6300) & ZymoBIOMICS™
Microbial Community DNA Standard (D6305), see Appendix C.
4
DNA is predominately 15-20 kb and amenable to Next-Generation Sequencing techniques requiring high
molecular weight DNA.
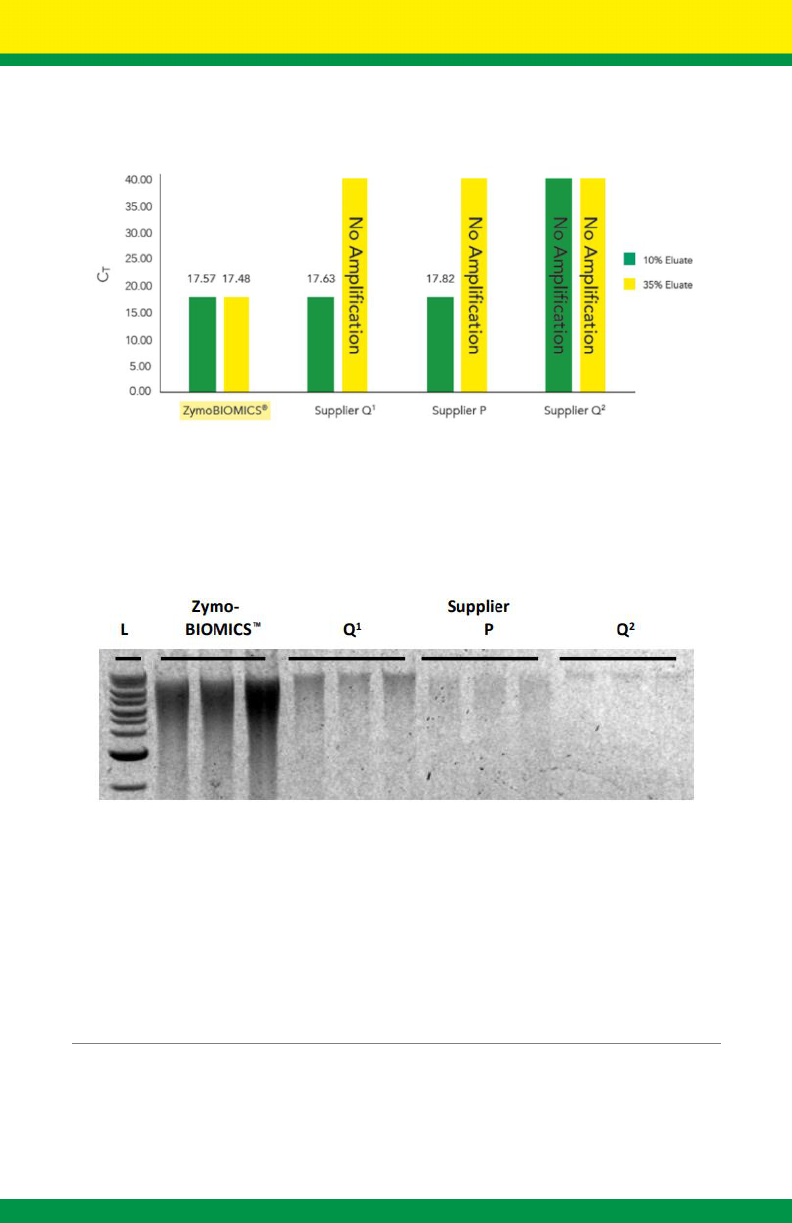
4
Ultra-pure DNA from Inhibitor Rich Samples
Figure 1. The ZymoBIOMICS™ DNA Miniprep Kit provides inhibitor-free DNA even when challenged
with extremely inhibitor rich samples. Real-time PCR was used to evaluate eluates recovered using the
ZymoBIOMICS™ DNA Miniprep Kit, and kits from Suppliers Q
1
, P, and Q
2
. Reaction volumes consisted
of either 10% or 35% of the eluate from each kit to detect the presence of PCR inhibitors. Each reaction
contained 25 ng of Brettanomyces DNA. Delayed and/or no amplification indicates PCR inhibition from
inefficient inhibitor removal.
Superior Yields
Figure 2. The ZymoBIOMICS™ DNA Miniprep Kit provides superior yields when compared to Suppliers
Q
1
, P, and Q
2
. 80 mg of feces was processed using each kit according to the manufacturers’ recommended
protocol. DNA was eluted using 100 µl ZymoBIOMICS™ DNase/RNase Free Water. 6 µl of each sample
was visualized in a 1.0% (w/v) agarose/ethidium bromide gel. Samples were processed in triplicate. L is
a 1Kb ladder.
Zymo Research offers a full suite of ZymoBIOMICS™ Services for reliable, accurate microbial and metagenomic
analyses.
Services include: Microbial Composition Profiling, Novel Microbe Identification, and Customizable Bioinformatics.
For details visit us at: http://www.zymoresearch.com/services/metagenomics
Or contact us at: services@zymoresearch.com

5
A) Bias Free Microbial DNA Extraction Using ZymoBIOMICS™ DNA Miniprep Kit
Validated with the ZymoBIOMICS™ Microbial Community Standard
B) Bias Free Microbial DNA Extraction Using ZymoBIOMICS™ DNA Miniprep Kit
From Human Stool
Figure 3. A) The ZymoBIOMICS™ DNA Miniprep Kit provides unbiased representation of the
organisms extracted from the ZymoBIOMICS™ Microbial Community Standard. DNA was extracted
from ZymoBIOMICS™ Microbial Community Standard using four different DNA extraction methods
(ZymoBIOMICS™ DNA Miniprep Kit, Human Microbiome Project Protocol, Supplier Q
1
, and Supplier Q
2
)
and analyzed using 16S rRNA gene sequencing. 16S rRNA genes were amplified with primers targeting
v3-4 region and the amplicons were sequenced on Illumina
®
MiSeq™ (2 x 250 bp). Overlapping paired-
end reads were assembled into complete amplicon sequences. The composition profile was determined
based on sequence counts after mapping amplicon sequences to the known 16S rRNA genes of the eight
different bacterial species.
B) The ZymoBIOMICS™ DNA Miniprep Kit reliably isolates DNA from even the toughest to lyse
Gram positive organisms, enabling unbiased analyses of microbial community compositions.
There is a significant increase in yield and Gram-positive bacterial abundance when DNA was isolated
using the ZymoBIOMICS™ DNA Miniprep Kit. Correlated with the results in Figure 3A, it can be concluded
that unbiased DNA isolation was achieved. DNA was extracted from 200 µl of human feces suspended in
PBS (10 % m/v) using four different DNA extraction methods (ZymoBIOMICS™ DNA Miniprep Kit, Human
Microbiome Project Protocol, Supplier Q
1
, and Supplier Q
2
) and analyzed using 16S rRNA gene
sequencing. 16S rRNA genes were amplified with primers targeting v3-4 region and the amplicons were
sequenced on Illumina
®
MiSeq™ (2 x 250 bp). Overlapping paired-end reads were assembled into
complete amplicon sequences. Amplicon sequences were profiled with Qiime using Greengenes 16S
rRNA gene database (gg_13_8).

6
Protocol
1. Add sample to a ZR BashingBead™ Lysis Tubes (0.1 & 0.5 mm).
Add 750 µl ZymoBIOMICS™ Lysis Solution to the tube and cap
tightly.
Note: For samples stored and lysed in DNA/RNA Shield™ Lysis Tubes, do
not add ZymoBIOMICS™ Lysis Solution and proceed to Step 2.
Sample Type
Maximum Input
Feces
200 mg
Soil
250 mg
Liquid Samples
1
and Swab Collections
2
250 µl
Cells (isotonic buffer, e.g. PBS)
50-100 mg (wet weight)
(10
9
bacterial and 10
8
yeast cells)
Samples in DNA/RNA Shield™
,3
≤ 1 ml
2. Secure in a bead beater fitted with a 2 ml tube holder assembly and
process using optimized beat beating conditions (speed and time) for
your device (see Appendix D)
4
.
Optional Stopping
Point: Following Step 2 is the best stopping point if breaking
up the work is needed. Samples can be stored post-lysis for several hours at room
temperature, or frozen at – 80 °C for long term storage.
3. Centrifuge the ZR BashingBead™ Lysis Tubes (0.1 & 0.5 mm) in a
microcentrifuge at ≥ 10,000 x g for 1 minute.
4. Transfer up to 400 µl supernatant to the Zymo-Spin™ III-F Filter in a
Collection Tube and centrifuge at 8,000 x g for 1 minute. Discard the
Zymo-Spin™ III-F Filter.
1
For water samples, filter using desired filter (not provided). Cut the filter into small pieces and place into ZR
BashingBead™ Lysis Tubes (0.1 & 0.5 mm).
2
Swabs can also be cut or broken, then placed directly in bead beating tube. For more information on processing
swab samples, see Appendix B.
3
Up to 1 ml of sample in DNA/RNA Shield can be processed directly in ZR BashingBead™ Lysis Tube. Adjust
final volume to 1 ml with ZymoBIOMICS™ Lysis Solution or DNA/RNA Shield, if necessary.
4
For optimal lysis efficiency and unbiased profiling all bead beater devices beyond those validated by Zymo
Research should be calibrated using the ZymoBIOMICS™ Microbial Community Standard. See Appendix C.

7
5. Add 1,200 µl of ZymoBIOMICS™ DNA Binding Buffer to the filtrate
in the Collection Tube from Step 4. Mix well.
6. Transfer 800 µl of the mixture from Step 5 to a Zymo-Spin™ IICR
Column in a Collection Tube and centrifuge at 10,000 x g for 1 minute.
7. Discard the flow through from the Collection Tube and repeat Step 6.
8. Add 400 µl ZymoBIOMICS™ DNA Wash Buffer 1 to the Zymo-
Spin™ IICR Column in a new Collection Tube and centrifuge at 10,000
x g for 1 minute. Discard the flow-through.
9. Add 700 µl ZymoBIOMICS™ DNA Wash Buffer 2 to the Zymo-
Spin™ IICR Column in a Collection Tube and centrifuge at 10,000 x g
for 1 minute. Discard the flow-through.
10. Add 200 µl ZymoBIOMICS™ DNA Wash Buffer 2 to the Zymo-
Spin™ IICR Column in a Collection Tube and centrifuge at 10,000 x g
for 1 minute.
11. Transfer the Zymo-Spin™ IICR Column to a clean 1.5 ml
microcentrifuge tube and add 100 µl (50 µl minimum)
ZymoBIOMICS™ DNase/RNase Free Water directly to the column
matrix and incubate for 1 minute. Centrifuge at 10,000 x g for 1 minute
to elute the DNA
5, 6
.
12. Place a Zymo-Spin™ III-HRC Filter in a new Collection Tube and add
600 µl ZymoBIOMICS™ HRC Prep Solution. Centrifuge at 8,000 x g
for 3 minutes.
13. Transfer the eluted DNA (Step 11) to a prepared Zymo-Spin™ III-HRC
Filter in a clean 1.5 ml microcentrifuge tube and centrifuge at exactly
16,000 x g for 3 minutes.
The filtered DNA is now suitable for PCR and other downstream
applications.
5
In some cases a brown-colored pellet may form at the bottom of the tube after centrifugation. Avoid this pellet
when collecting the eluted DNA.
6
If fungi or bacterial cultures were processed; the DNA is now suitable for all downstream applications.

8
Appendices
Appendix A
Sample Collection
For high quality reproducible microbiomics data, DNA/RNA Shield™ is
recommended for sample collection to avoid bias or erroneous results due
to compositional changes from nucleic acid degradation or microbial
growth. DNA/RNA Shield™ provides an unbiased molecular snapshot of
the sample at the time of collection by preserving nucleic acids at ambient
temperature and inactivating organisms including infectious agents.
Samples can be stored and transported easily and safely with DNA/RNA
Shield™ and is ideal for applications such as PCR, 16S rRNA gene
sequencing, and shotgun metagenomic sequencing. DNA/RNA Shield™
can preserve nucleic acids in nearly any sample including feces, soil,
saliva, blood, and tissues.
DNA/RNA Shield™ - Lysis Tube (Microbe) – Simply add sample, seal,
and store at ambient temperature. The Lysis Tube is immediately ready
for bead beating, thereby streamlining the collection to extraction
transition. (Cat. No. R1103)
DNA/RNA Shield™ – Fecal Collection Tube – The collection device is
specifically designed for easy collection and stabilization of feces. Includes
a scoop built for collecting 1 gram of feces (or any other sample such as
saliva or soil). (Cat. No. R1101)
DNA/RNA Shield™ – Swab Collection Tube – Easy collection of
biological samples; swab has breakable tip to allow for easy sample
collection and removes the need to dispose of a potentially biohazardous
swab material. (Cat. No. R1106 & R1107).

9
A) DNA/RNA Shield™ Preserves Nucleic
Acids at Room Temperature
Figure 4.
A) Nucleic acids in stool are
effectively stabilized in DNA/RNA
Shield™ at room temperature.
Graph shows spike-in DNA and RNA
controls from stool purified at the
indicated time points and analyzed by
(RT)qPCR. Controls: HSV-1 and HIV
(AcroMetrix™, Life Technologies).
B) Microbial composition of stool
is unchanged after one month at
ambient temperature with
DNA/RNA Shield™. Stool samples
suspended in DNA/RNA Shield™ and
stored at room temperature were
compared to stool without
preservative for one month. They
were sampled at the indicated time
points and processed with
ZymoBIOMICS™ DNA Miniprep Kit.
The extracted DNA was then
subjected to microbial composition
profiling via 16S rRNA gene targeted
sequencing. Graphs show both
phylum composition (left) and genus
composition (right). Samples stored
with DNA/RNA Shield™ had a
constant microbial composition while
the samples stored without shifted
dramatically.
C) Viruses, bacteria and yeast are
effectively inactivated by DNA/RNA
Shield™. Samples containing the
infectious agent (viruses, bacteria,
yeast) were treated with DNA/RNA
Shield™ or mock (PBS) treated for 5
minutes. Titer (PFU) was
subsequently determined by plaque
assay. Validated by: Influenza A - D.
Poole and Prof. A. Mehle, Department
of Medical Microbiology and
Immunology, University of Wisconsin,
Madison; Ebola (Kikwit) - L. Avena
and Dr. A. Griffiths, Department of
Virology and Immunology, Texas
Biomedical Research Institute; HSV-
1/2.
B) DNA/RNA Shield™ Preserves Microbial
Composition at Room Temperature
C) DNA/RNA Shield
™
Inactivates Pathogens
for Safe Transport and Storage

10
Appendix B
Application Notes
DNA/RNA Shield™ Lysis Tubes (Microbe) (Cat. No. R1103)
1. Collect sample directly into the DNA/RNA Shield™ Lysis Tube
(Microbe).
2. Directly proceed to Step 2 of the protocol (page 6) and bead beat in
the DNA/RNA Shield™ Lysis Tube (Microbe) according to provided
recommendations.
3. Proceed with the remaining protocol as written.
DNA Viruses
For unbiased metagenomics analysis of viruses, incorporating a
Proteinase K digestion prior to bead beating is recommended.
1. Following Step 2 (page 6) add 5% (v/v) of Proteinase K (Cat. No.
D3001-2-5) to the lysate within the ZR BashingBead™ Lysis Tubes
(0.1 & 0.5 mm) and incubate for 30 minutes at 55˚C.
2. Proceed to Step 3 (page 6) and continue with the remaining protocol
as written.
Cheese and Protein Rich Biofluids (e.g. Milk, Sputum, Saliva, Spinal
Fluid, Blood, and Serum)
1. Add ≤ 0.4 g of cheese or ≤ 200 µl of biofluid to the ZR BashingBead™
Lysis Tubes (0.1 & 0.5 mm). Add 750 µl of ZymoBIOMICS™ Lysis
Solution.
2. Add 20 µl of Proteinase K (20 µg/µl) (cat. no. D3001-2-5) to the
ZymoBIOMICS™ Lysis Tubes (0.1 & 0.5 mm) and incubate for 30
minutes at 55˚C.
3. Continue to Step 2 (page 6) and proceed with the protocol as written.
Plant Tissue (Leaves and other plant material)
Plant tissues such as leaves and roots contain DNA sources within the
host tissue that can overwhelm 16S rRNA gene targeted sequencing (from
both mitochondria & chloroplast). Microbes must be removed from the
plant surface to exclude host tissue from the bead beating process.

11
(A) Plant tissue – Centrifugation of cells
1. Suspend plant tissue in isotonic solution (e.g. PBS) and gently
sonicate or vortex briefly.
2. Remove plant tissue from solution and centrifuge at 15,000 x g for 10
minutes to pellet the cells.
3. Without disturbing the pellet, slowly decant or pipette out the
supernatant, leaving behind 100 – 300 µl of pellet.
4. Add ZymoBIOMICS™ Lysis Solution to the cells to a final volume
of 1 ml and mix to resuspend. Transfer the mixture to the ZR
BashingBead™ Lysis Tubes (0.1 & 0.5 mm) and proceed to Step
2 (page 6).
(B) Plant tissue – Filtration of cells
1. Place plant tissue in a submerging volume of PBS inside of a conical
tube and gently sonicate or vortex briefly. Remove plant tissue from
liquid volume.
2. Filter liquid using a 0.22 µm filter (not provided).
3. Cut the filter and place directly into the ZR BashingBead
™
Lysis
Tubes (0.1 & 0.5 mm) and proceed to Step 1 (page 6).
(C) Plant root – Lysis of surface microbes
1. Cut root into small pieces and place directly into ZR BashingBead
™
Lysis Tubes (0.1 & 0.5 mm) with 750 µl of ZymoBIOMICS
™
Lysis
Buffer.
2. Lysis should be performed with a lower speed bead beating device
(e.g. vortex adapter for 20 minutes) to avoid the host tissue
contamination.
3. Continue to Step 3 (page 6) and proceed with the remaining protocol
as written.
Water/Air Samples
1. Filter samples using desired filter (not provided) prior to Step 1 (page
6).
2. Cut the filter into small pieces and place them inside the ZR
BashingBead
™
Lysis Tubes (0.1 & 0.5 mm) and add 750 µl of
ZymoBIOMICS Lysis Solution.
3. Continue to Step 2 (page 6) and proceed with the remaining protocol
as written.

12
Lytic Enzymes
Lytic enzymes, such as Lysozyme, Lysostaphin, MetaPolyzyme, etc. can
be used with this kit using the following:
(A) Enzymatic lysis followed by bead beating:
1. Perform enzymatic digestion under manufacturer’s recommended conditions
(temperature/time/concentration).
Note: If sample is stored in DNA/RNA Shield, perform the following:
a. Centrifuge sample at ≥ 10,000 x g for 1 minute.
b. Transfer supernatant to a ZR BashingBead Lysis Tube (0.1 & 0.5 mm), to be
used in Step 2, below.
c. Re-suspend pellet in a buffer suitable for enzymatic treatment (ex. PBS or other
isotonic solution).
2. Transfer the digestion mixture to a ZR BashingBead™ Lysis Tube (0.1 &
0.5 mm).
3. Add 750 µl ZymoBIOMICS™ Lysis Solution.
Note: For samples in DNA/RNA Shield, raise to a final volume of 1 ml with DNA/RNA
Shield.
4. Proceed to Step 2 (page 6) and continue with the remaining protocol as
written.
(B) Enzymatic lysis only (no bead beating):
1. Perform enzymatic digestion under manufacturer’s recommended conditions
(temperature/time/concentration).
Note: If sample is stored in DNA/RNA Shield, perform the following:
a. Centrifuge sample at ≥ 10,000 x g for 1 minute.
b. Transfer supernatant to a clean microcentrifuge tube, to be used in Step 2.
c. Re-suspend pellet in a buffer suitable for enzymatic treatment (ex. PBS or other
isotonic solution).
2. Raise the volume of sample to 400 µl with ZymoBIOMICS™ Lysis Solution.
3. Continue to Step 4 (page 6) and proceed with the remaining proceed as
written.

13
Hair, Feather, and Nail Samples:
1. To ≤ 25 mg sample, add 90 µl Water, 90 µl Solid Tissue Buffer (Blue)
(Cat. No. D4068-2-6), 10 µl 1M DTT, and 10 µl Proteinase K (Cat.
No. D3001-2-5) in a microcentrifuge tube.
2. Mix thoroughly or vortex 10-15 seconds and then incubate the tube at
55
º
C overnight.
3. Transfer lysate to a ZR BashingBead
™
Lysis Tube (0.1 & 0.5 mm)
and then add 750 µl ZymoBIOMICS™ Lysis Solution.
4. Continue to Step 2 (page 6) and proceed with the remaining protocol
as written.
Tissue and Insect Samples
Tissue and Insect samples can be processed three different ways,
depending on the sample type and the equipment available. The
recommendations are listed next to the options below:
(A) Proteinase K - Tissue
1. Add up to 15 mg of tissue to a 1.5 ml microcentrifuge tube, then add a
solution of 95 µl water, 95 µl Solid Tissue Buffer (Blue) (Cat. No. D4068-2-
6) and 10 µl Proteinase K (Cat. No. D3001-2-5). Incubate for at least 1 hour
at 55° C or until tissue clarifies (samples can be incubated overnight without
affecting DNA quality).
2. Transfer digestion to a ZR BashingBead™ Lysis Tube (0.1 & 0.5 mm) and
add 750 µl of ZymoBIOMICS™ Lysis Solution.
3. Proceed to Step 2 (page 6) and continue with the protocol as written.
(B) Bead beating - Tissue and Insect
1. Add up to 15 mg of tissue/insect sample in a ZR BashingBead
™
Lysis Tube
(2.0 mm) (Cat. No. S6003-50) with 750 µl of ZymoBIOMICS
™
Lysis
Solution.
2. Secure in a bead beater fitted with a 2 ml tube holder assembly and process
at maximum speed for ≥ 5 minutes.
Note: Processing time will vary based on sample input and bead beater. Times
may be as little as 5 minutes when using high-speed cell disrupters (FastPrep
®
-
24) or as long as 20 minutes when using lower speeds (e.g., Vortex Genie
®
).
3. Transfer the entire lysate to the ZR BashingBead™ Lysis Tube (0.1 & 0.5
mm), proceed to Step 2 (page 6), and continue with protocol as written.
(C) Mortar & Pestle - Tissue and Insect
1. Homogenize up to 15 mg tissue/insect sample with a mortar and pestle while
submersed in liquid nitrogen.
2. Transfer the entire sample into the ZR BashingBead™ Lysis Tube (0.1 &
0.5 mm) and add 750 µl of ZymoBIOMICS™ Lysis Solution.
3. Proceed to Step 2 (page 6) and continue with the protocol as written.

14
Samples Collected with Swabs
(A) Directly process swab
1. Directly break swab at breakpoint or cut the swab into a ZR BashingBead
Lysis Tube (0.1 & 0.5 mm).
2. Proceed to Step 1 (page 6) and continue with the protocol as written.
(B) Indirectly process swab
1. Vortex the swab in the ZR BashingBead™ Lysis Tube (0.1 & 0.5 mm) with
750 µl of ZymoBIOMICS™ Lysis Solution for 30 seconds to transfer the
microbes into solution.
2. Remove the swab and proceed to bead beating in Step 2 (page 6).
Figure 5. Phylum composition of a
simulated microbial community when
bead beating was performed with and
without the presence of a Puritan
HydraFlock
®
sterile flocked collection
device placed in a ZR BashingBead
Lysis Tube and processed at
maximum speed (6.5 m/s) for 5
minutes. The extracted DNA was then
subjected to microbial composition
profiling via 16S rRNA gene targeted
sequencing. Experiment was
performed in technical duplicates.
Bacterial Endospore Lysis
ZymoBIOMICS DNA Miniprep Kit is capable of effectively lysing bacterial
endospores, and also achieves higher yield when compared to
competition.
Figure 6. DNA Extractions were
performed using the ZymoBIOMICS
®
DNA Kit and DNeasy PowerSoil with 6
x 10
8
B. subtilis CFU. Dneasy
PowerSoil recovered 0.62 ng/µl DNA,
while the ZymoBIOMICS
®
DNA Kit was
capable of recovering 9.67 ng/µl in a
50 µl elution volume. Extractions were
performed in triplicate and quantified
via Qubit.

15
Urine
(A) Pelleting cells from fresh/frozen urine
1. Pellet the bacterial cells by centrifuging the urine at 15,000 x g for 10 minutes.
2. Without disturbing the pellet, slowly decant or pipette out the supernatant,
leaving behind 100 – 400 µl of pellet.
3. Add ZymoBIOMICS
™
Lysis Solution to a final volume of 800 µl and then
transfer the mixture to a ZR BashingBead
™
Lysis Tube (0.1 & 0.5 mm).
Proceed to Step 2 (page 6) and continue with the protocol as written.
(B) Pelleting cells from stabilized urine
1. Add 70 µl Urine Conditioning Buffer (Cat. No. D3061-1-140) for every 1 ml
of urine and mix well by vortexing.
Note: Urine stabilized by the Urine Conditioning Buffer can be stored for up to 1 month
at ambient temperature. When samples are ready to be processed, mix well by
vortexing, and proceed to Step 2.
2. Centrifuge at 3,000 x g for 15 minutes.
3. Without disturbing the pellet, slowly decant or pipette out the supernatant,
leaving behind 100 – 400 µl of pellet.
4. Add ZymoBIOMICS
™
Lysis Solution to a final volume of 800 µl and then
transfer the mixture to a ZR BashingBead
™
Lysis Tube (0.1 & 0.5 mm).
Proceed to Step 2 (page 6) and continue with the protocol as written.
Figure 7. Phylum composition of urine preserved in Urine Conditioning Buffer™ (UCB™), which
preserves the microbial composition of urine with simulated stool contamination for a month at room
temperature. Urine with UCB™ added (Zymo Research, D3061-1-160) was stored at room
temperature and analyzed over a month period. At the indicated time points (0 Days, 2 weeks, and 1
month), DNA was extracted using the ZymoBIOMICS
™
DNA Miniprep Kit. The extracted DNA was
then subjected to microbial composition profiling via 16S rRNA gene targeted sequencing. Experiment
was performed in technical duplicates.

16
Appendix C
Standardize Sample Preparation with ZymoBIOMICS
™
Microbial
Community Standards
The ZymoBIOMICS
™
Microbial Community Standard (Cat. No. D6300)
is a mock microbial community of defined and well characterized
composition making it the perfect control for all microbiome profiling and
metagenomics analyses.
It is ideal for assessing bias of DNA extraction methods since it contains
three easy-to-lyse Gram-negative bacteria (e.g. Escherichia coli), five
tough-to-lyse Gram-positive bacteria (e.g. Listeria monocytogenes), and
two tough-to-lyse yeasts (e.g. Saccharomyces cerevisiae).
Bead Beating Device Calibration Protocol:
Zymo Research suggests calibrating bead beating devices with the
ZymoBIOMICS
™
Microbial Community Standard in order to ensure bias
free microbial extraction. For lysis on the Vortex Genie
, we suggest a
time course ranging from 10-45 minutes with the vortex at maximum
speed. For high-speed cell disruptors such as the MP FastPrep
-24 we
suggest a time course at maximum speed with a range of 3-10 minutes.
The resulting DNA should be evaluated by quantifying DNA yield and
changes in microbial profile at each time point. The bead beating time that
yields a profile that closely matches the theoretical composition should
become standard operating procedure for the bead beating device.
ZymoBIOMICS
™
Microbial Community DNA Standard (Cat. No.
D6305) is a mixture of genomic DNA extracted from pure cultures of eight
bacterial and two fungal strains. Genomic DNA from each culture was
quantified before mixing. The ZymoBIOMICS
™
Microbial Community
Standard allows for assessment of bias from library preparation,
sequencing, and bioinformatics analysis.
It serves perfectly as a microbial standard for benchmarking the
performance of microbiomics or metagenomics analyses, including those
provided by a 3
rd
party.
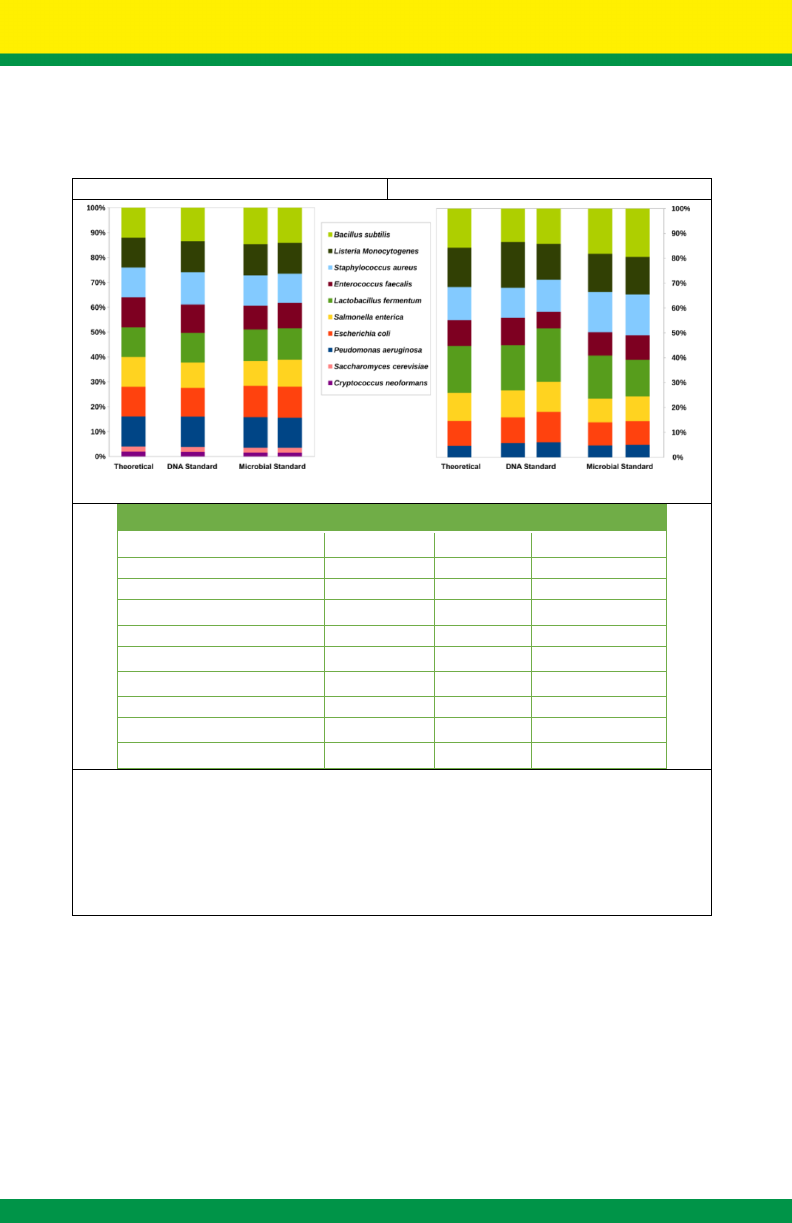
17
Accurate composition for reliable use to evaluate shotgun seq. and
16S rRNA seq.
gDNA by Shotgun Sequencing
16S Counts by 16S Sequencing
Species
GC %
Gram Stain
gDNA Abun. (%)
Pseudomonas aeruginosa
66.2
-
12
Escherichia coli
56.8
-
12
Salmonella enterica
52.2
-
12
Lactobacillus fermentum
52.8
+
12
Enterococcus faecalis
37.5
+
12
Staphylococcus aureus
32.7
+
12
Listeria monocytogenes
38.0
+
12
Bacillus subtilis
43.8
+
12
Saccharomyces cerevisiae
38.4
Yeast
2
Cryptococcus neoformans
48.2
Yeast
2
Figure 8. Characterization of the microbial composition of the two ZymoBIOMICS
™
standards with
shotgun metagenomic sequencing (left panel) and 16S rRNA gene targeted sequencing (right panel).
The measured composition of the two standards agrees with the theoretical/designed composition.
“DNA Standard” represents ZymoBIOMICS
™
Microbial Community DNA Standard (DNA version) and
“Microbial Standard” represents ZymoBIOMICS
™
Microbial Community Standard (cellular version).
Genomic DNA composition by shotgun sequencing was calculated based on counting the amounts
of raw reads mapped to each genome. 16S composition by 16S rRNA gene targeted sequencing
was calculated based on counting the amount of 16S raw reads mapped to each genomes.

18
A) Use ZymoBIOMICS
™
Microbial Standards for assessing GC-Bias in
Shotgun Metagenomics
B) Perfect for tracking PCR Chimera in 16S rRNA Gene Sequencing
Figure 9.
A) Library preparation for shotgun metagenomic sequencing was performed in two different ways: one
by supplier I and one by an in-house method. Shotgun sequencing was performed on Illumina
®
MiSeq
™
with paired-end sequencing (2 x 150 bp). Raw reads were mapped to the 10 microbial
genomes to evaluate the potential effect of GC content on sequencing coverage. Normalized
coverage was calculated by normalization by the average sequencing coverage of each genome.
B) PCR chimera increases with PCR cycle number in the library preparation process of 16S rRNA gene
targeted sequencing. 20 ng ZymoBIOMICS
™
Microbial Community Standard was used a template.
The PCR reaction was performed with ZymoBIOMICS
™
PCR Premix and with primers that target v3-
4 region of 16S rRNA gene. Chimera rate in percentage was determined with Uchime and using the
16S rRNA gene of the 8 bacterial strains in the standard as reference PCR.

19
Appendix D
Optimized Lysis Protocols for Bead-Beating
The following conditions with different mechanical lysis machines were validated
with minimum bias using the ZymoBIOMICS
™
Microbial Community Standard.

20
Troubleshooting
For Technical Assistance, please contact 1-888-882-9682 or E-mail
tech@zymoresearch.com.
Problem
Possible Causes and Suggested Solutions
Background
Contamination
• Clean workspace, centrifuge, and pipettes with 10%
bleach to routinely to avoid contamination.
• Use of kit in exposed environment without proper
filtration. Check pipettes, pipette tips, microcentrifuge
tubes, workspace, etc. for contamination.
• Make sure bags of columns and buffer bottles are
properly sealed for storage. Use of these outside a clean
room or hood can result in contamination.
Low DNA
Yield
Lysis Methods
• Refer to Appendix D for validated bead beating devices
and parameters.
• Bead beating devices that oscillate in a single dimension
(only vertically or only horizontally) have been observed
to inefficiently lyse very recalcitrant species. Devices that
oscillate three-dimensionally or in a figure-8 motion often
lyse microbes efficiently.
Incomplete Debris Removal
• For high density samples, ensure lysate is centrifuged
properly to pellet insoluble debris following bead beating.
Ensure that none of the debris is transferred to the Zymo-
Spin™ III-F Filter in the next step.

21
Low DNA Yield
(cont.)
Input
• If the lysate does not pass through the column or is
extremely viscous, use less input material. Too much
sample input can cause cellular debris to overload the
column and insufficient flow.
• Consult the Sample Input Table on Page 6 for
information on your particular input limit based on
sample.
Binding Step
• Ensure that the ZymoBIOMICS™ DNA Binding Buffer
is completely mixed with lysate before loading onto the
column. Improperly mixed samples can lead to poor
DNA recovery.
Elution Procedure
• Ensure the ZymoBIOMICS™ DNase/RNase Free Water
hydrates the matrix for at least 1 minute before
centrifugation.
• To increase yields, heat the ZymoBIOMICS™
DNase/RNase Free Water to 60ºC before use.
Additionally, users can reload the eluate onto the column
matrix, incubate at room temperature for 3 minutes, and
centrifuge again to increase yield without further dilution.

22
Ordering Information
Product Description
Catalog No.
Size
ZymoBIOMICS™ DNA Microprep Kit
D4301
50 Preps.
ZymoBIOMICS™ DNA Microprep Kit (Lysis Matrix Not Included)
D4305
50 Preps.
ZymoBIOMICS™ DNA Miniprep Kit
D4300T
5 Preps.
ZymoBIOMICS™ DNA Miniprep Kit
D4300
50 Preps.
ZymoBIOMICS™ DNA Miniprep Kit (Lysis Matrix Not Included)
D4304
50 Preps.
ZymoBIOMICS™-96 DNA Kit (Includes BashingBead™ Lysis
Rack)
D4303
2 x 96 Preps.
ZymoBIOMICS™-96 DNA Kit (Includes BashingBead™ Lysis
Tubes)
D4309
2 x 96 Preps.
ZymoBIOMICS™-96 DNA Kit (Lysis Matrix Not Included)
D4307
2 x 96 Preps.
ZymoBIOMICS™-96 Magbead DNA Kit (Includes BashingBead™
Lysis Rack)
D4302
2 x 96 Preps.
ZymoBIOMICS™-96 Magbead DNA Kit (Includes BashingBead™
Lysis Tubes)
D4308
2 x 96 Preps.
ZymoBIOMICS™-96 Magbead DNA Kit (Lysis Matrix Not Included)
D4306
2 x 96 Preps.

23
Individual Kit Components
Catalog No.
Amount
ZR BashingBead
™
Lysis Tubes (0.1 & 0.5 mm)
S6012-50
50 Tubes
ZymoBIOMICS
™
Lysis Solution
D4300-1-40
40 ml
ZymoBIOMICS
™
DNA Binding Buffer
D4300-2-100
100 ml
ZymoBIOMICS
™
DNA Wash Buffer 1
D4300-3-50
50 ml
ZymoBIOMICS
™
DNA Wash Buffer 2
D4300-4-60
60 ml
ZymoBIOMICS
™
DNase/RNase Free Water
D4302-5-10
10 ml
Zymo-Spin™ IICR Columns
C1078-50
50 Pack
Zymo-Spin ™ III-F Filters
C1057-50
50 Pack
OneStep™ PCR Inhibitor Removal Kit
D6030
50 Preps.
Collection Tubes
C1001-50
C1001-500
C1001-1000
50 Pack
500 Pack
1,000 Pack
Sample Collection
Catalog No.
Amount
DNA/RNA Shield
™
- Lysis Tube (Microbe)
R1103
50 Pack
DNA/RNA Shield
™
– Fecal Collection Tube
R1101
10 Pack
DNA/RNA Shield
™
– Swab and Collection Tube
R1106
R1107
10 Pack
50 Pack
DNA/RNA Shield
™
R1100-50
R1100-250
50 ml
250 ml

24
Explore Other Microbiome Products
✓ To collect and transport samples at ambient temperatures:
DNA/RNA Shield
™
and Collection Devices
1X Reagent #R1100
For sample lysis and stabilization of
DNA/RNA
2X Concentrate #R1200
Reagent concentrate (2X) for use with
liquids at 1:1 ratio
Fecal Collection Tube #R1101
15 mL container (prefilled with 9 mL
DNA/RNA Shield
™
). Direct collection of
up to 1g or 1 mL stool
Collection Tube w/ Swab #R1106
12 x 80 mm screwcap container filled
with 1 mL DNA/RNA Shield
™
and sterile
swab for specimen collection
✓ Streamlined workflows with comprehensive bioinformatics analysis and publication-
ready plots and figures:
ZymoBIOMICS
™
Services
Targeted Sequencing Service 16S #Q2001
With DNA Extraction
Targeted Sequencing Service 16S #Q2012
Without DNA Extraction
Targeted Sequencing Service ITS #Q2003
With DNA Extraction
Targeted Sequencing Service ITS #Q2003
Without DNA Extraction
✓ Microbial standards and references for profiling quality control, benchmarking, positive
controls, and to assess performance of entire microbiomic/metagenomic workflows:
ZymoBIOMICS
™
Standards and Reference Materials
Microbial Community Standard
#D6300
Contains 8 bacteria and 2 yeasts
for QC and method optimization
Microbial Community DNA Standard
#D6305
Contains 8 bacteria and 2 yeasts
DNA for bioinformatics optimization
Gut Microbiome Standard #D6331
Contains 21 different human gut
strains for method benchmarking
Fecal Reference with TruMatrix
™
Technology #D6323
Contains real human fecal
material for benchmarking and
improved data reproducibility

25
100% satisfaction guarantee on all Zymo Research products,
or your money back.
Zymo Research is committed to simplifying your research with quality products
and services. If you are dissatisfied with this product for any reason, please call
1(888) 882-9682.
Integrity of kit components is guaranteed for up to one year from date of purchase.
Reagents are routinely tested on a lot-to-lot basis to ensure they provide the highest performance and reliability.
This product is for research use only and should only be used by trained professionals. It is not for use in
diagnostic procedures. Some reagents included with this kit are irritants. Wear protective gloves and eye
protection. Follow the safety guidelines and rules enacted by your research institution or facility.
™ Trademarks of Zymo Research Corporation, ® Registered Trademarks of Zymo Research Corporation
Other trademarks: Vortex Genie® (Scientific Industries, Inc.), FastPrep® (MP Biomedicals), Precellys® (Bertin
Technologies, Inc.), Illumina® MiSeq™, (Illumina Inc.), AcroMetrix™, Qubit® (Thermo Fisher Scientific Inc.),
DNeasy® (Qiagen), Hydraflock® (Puritan Medical Products), Bead Ruptor
®
(Omni International)

